Myopathy is a congenital pathology caused by a gene mutation. The mechanism of development of the disease is not fully understood, so it is not possible to predict the likelihood of having a sick child. It happens that two absolutely healthy people give birth to a child with some form of myopathy. It has been established that the cause is a metabolic disorder in muscle tissue, as a result of which they lose creatine and become depleted.
Becker myopathy, or benign pseudohypertrophic myopathy, is the mildest form of the disease, which was first described in 1955 by Becker and Keener. The genetic similarity of Becker and Duchenne myopathy has been established - both varieties cause allelic mutations of the same genes. A characteristic feature of Becker's dystrophy is that only males are affected; there is 1 affected child per 20 thousand newborns.
Duchenne-Becker muscular dystrophy
OMIM 310200
Our team of professionals will answer your questions
Duchenne muscular dystrophy (DMD) is a hereditary disease that begins at the age of 2-5 years and is characterized by progressive muscle weakness, atrophy and pseudohypertrophy of the proximal muscles , often accompanied by cardiomyopathies and intellectual impairment. In the early stages of the disease, increased fatigue when walking and changes in gait (“duck walk”) are observed. In this case, gradual degradation of muscle tissue occurs. 95% of patients stop walking at the age of 8-12 years. At the age of 18-20 years, patients usually die, often from respiratory failure. There is an allelic form of DMD - Becker muscular dystrophy (BMD, OMIM 310200), which is characterized by similar clinical manifestations, a later onset (at about 10-16 years) and a milder course. Such patients often retain the ability to walk up to 20 years, and some - up to 50-60 years, although the same muscles are involved in the pathological process as in DMD. The life expectancy of such patients is reduced slightly.
A biochemical marker of the disease is an increased (100-200) times the level of creatine phosphokinase ( CPK ) in the blood. In carriers of the damaged gene, the level of CPK on average is also slightly increased.
The type of inheritance of Duchenne muscular dystrophy is X-linked recessive, i.e. it affects almost exclusively boys, while women with a damaged gene on one of the X chromosomes are carriers of DMD. But in rare cases, girls can also suffer from Duchenne muscular dystrophy. The reasons for this may be the preferential inactivation of the X chromosome with a normal allele in heterozygous carriers of the mutant DMD gene, X-autosomal translocation affecting this gene, hemizygosity for the mutant allele and the presence of phenocopies (diseases associated with disruption of other proteins included in the dystrophin-glycoprotein complex ). In approximately 2/3 of cases, the son receives a damaged chromosome from the carrier mother; in other cases, the disease occurs as a result of a de novo mutation in the germ cells of the mother or father, or in the precursors of these cells. Duchenne muscular dystrophy (DMD) occurs in approximately one in 2,500 to 4,000 male births.
The DMD gene, responsible for progressive Duchenne/Becker muscular dystrophy (DMD/BMD), is located in the Xp21.2 locus and has a size of 2.6 million bp. and consists of 79 exons. In 60% of cases, mutations leading to DMD/BMD are long deletions (from one to several dozen exons), in 30% of cases they are point mutations, and in 10% of cases they are duplications. Due to the presence of so-called deletion hot spots, amplification of the 27 exons and promoter region of the DMD gene allows detection of approximately 98% of all large deletions. The search for point mutations is difficult due to the large size of the gene and the absence of major mutations.
The Center for Molecular Genetics carries out measurements of CPK levels in the blood, as well as direct diagnosis of DMD/BMD, which is a search for large deletions/duplications in all exons of the DMD gene and a search for “point” mutations of the DMD gene using NGS (next generation sequencing). NGS research also makes it possible to detect deletions of all exons of the DMD gene in sick boys. Analysis of all exons of a gene allows us to determine the exact exon boundaries of the deletion in the case of its detection, and thus determine whether this deletion leads to a shift in the protein reading frame, which in turn is important for predicting the form of the disease - Duchenne or Becker muscular dystrophy. Thus, a combination of various research methods makes it possible to detect almost all mutations of the DMD gene.
The presence of any type of mutation (deletions/duplications in one or more exons, “point” mutations) is a molecular genetic confirmation of the clinical diagnosis of Duchenne/Becker muscular dystrophy and allows for prenatal diagnosis in a given family.
Attention! To measure CPK levels, the blood must be fresh (not frozen)!
In the case of prenatal diagnosis, fetal biomaterial is required, which can be chorionic villi (from the 8th to 12th week of pregnancy), amniotic fluid (from the 16th to 24th week of pregnancy) or umbilical cord blood (from the 22nd week of pregnancy). weeks of pregnancy).
We have developed kits for DNA diagnostics of progressive Duchenne/Becker muscular dystrophy. The kits are intended for use in molecular genetic diagnostic laboratories.
ICD-10 code
Myocardial dystrophy is understood as a specific heart disease that is not inflammatory in nature. It is characterized by metabolic disorders that occur in myocytes. At the same time, the contractility of the heart muscle changes, and heart failure appears.
Classification of diseases ICD-10 is a normative document that contains codes for diseases systematized by sections. This is an international development that facilitates the collection and storage of information to create statistics for all countries. ICD-10 was created by the World Health Organization. The document is revised every 10 years and additions are always made to it. If previously there was a separate code for myocardial dystrophy, now it is not assigned to this disease according to ICD-10. However, doctors may give the number I.42, which implies cardiomyopathy. Despite the similarity of names, cardiomyopathy and myocardial dystrophy are considered different phenomena, since the first involves a wider range of different cardiac pathologies that are not associated with heart disease and the vascular system. Therefore, these terms cannot be used as synonyms. But for myocardial dystrophy there is still one code according to ICD-10, which assumes only cardiomyopathy with metabolic disorders and nutritional problems.
In general, section I42 assumes cardiomyopathy, but excludes conditions that are complicated by pregnancy and postpartum ischemic disease. Number I42.0 suggests a dilated type, and 42.1 is a hypertrophic obstructive pathology. Number 42.2 refers to other forms of hypertrophic pathology. Code 42.3 is endomyocardial disease, and 42.4 is fibroelastosis of the endocardial type. If the patient has other restrictive cardiac pathologies, then the number is set to 42.5. If cardiomyopathy is of alcoholic origin, then code 42.6 is assumed. If the disease is caused by the influence of drugs or other external factors, then the number 42.7 is set. Other cardiomyopathies have the number 42.8, and if the forms of the pathology are not specified, then the code is 42.9.
Difference Between Duchenne and Becker Muscular Dystrophy
The key difference between Duchenne muscular dystrophy and Becker muscular dystrophy is that in Duchenne muscular dystrophy dystrophin is absent , whereas in Becker muscular dystrophy dystrophin is present, albeit at low levels. This is the key difference between Duchenne and Becker muscular dystrophy. Another important difference between these two conditions is their level of severity.
Duchenne muscular dystrophy and Becker muscular dystrophy are X-linked recessive disorders characterized by progressive proximal muscle weakness caused by muscle fiber degeneration and characterized by changes in dystrophin levels. Muscular dystrophies are inherited, progressive muscle disorders that result from defects in one or more genes essential for normal muscle structure and function. Duchenne dystrophy and Becker dystrophy are the second most common muscular dystrophy (after fascicapulohimeral muscular dystrophy). They are caused by mutations in the dystrophin gene, the largest known human gene, at the Xp21.2 locus. In Duchenne dystrophy, this mutation results in a severe lack of
- What is Duchenne muscular dystrophy
- What is Becker muscular dystrophy?
- Similarities between Duchenne and Becker muscular dystrophy
- What is the difference between Duchenne and Becker muscular dystrophy?
- Conclusion
What is Duchenne muscular dystrophy?
Duchenne muscular dystrophy, or Duchenne muscular dystrophy (DMD), is an X-linked recessive disorder characterized by progressive proximal muscle weakness caused by degeneration of muscle fibers and the absence of the structural rod-shaped protein dystrophin, which is essential for cell membrane stability. This disease is caused by mutations in the dystrophin gene, the largest known human gene, at the Xp21.2 locus. In Duchenne dystrophy, this mutation causes a reading frame shift and a severe loss of ( This disease affects one in three thousand male infants.
Duchenne muscular dystrophy
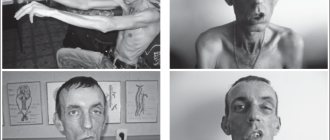
This disorder usually appears between 2 and 3 years of age. Weakness affects the proximal muscles, usually in the lower extremities. Children often walk, have a gait and lordosis. They have difficulty running, jumping, climbing stairs and even from the floor. Children fall frequently, often causing broken arms or legs (in approximately 20% of patients). The progression of weakness is steady, and limb flexion contractures and scoliosis develop in almost all children. Solid pseudohypertrophy develops (fatty and fibrous replacement of certain expanded muscle groups). Most children need to use a wheelchair by age 12 and end up dying from respiratory complications by age 20.
Consequences of damage to the heart muscle include dilated cardiomyopathy, conduction abnormalities, and arrhythmias. Such complications occur in about a third of patients aged 14 years and in all patients over 18 years of age. however, because these patients are unable to exercise, cardiac involvement is usually asymptomatic until late in the disease. About a third of patients have mild, nonprogressive intellectual impairment that affects language abilities more than productivity.
Mutation analysis of DNA from peripheral blood leukocytes is the main confirmatory test. It can detect abnormalities in the dystrophin gene (deletions in approximately 70% of patients with Duchenne dystrophy and in 85% of patients with Becker dystrophy; duplications in approximately 10% of both groups).
If genetic testing does not confirm the diagnosis, dystrophin testing with immunostaining of muscle biopsy specimens should be performed. Dystrophin is not detected in patients with Duchenne dystrophy.
Patients with Duchenne dystrophy should have a baseline assessment of cardiac function with ECG and echocardiography at the time of diagnosis or by age 6 years.
Carrier identification and prenatal diagnosis are possible using traditional testing (eg, pedigree analysis, CK determination, fetal sex determination) in combination with recombinant DNA analysis and muscle dystrophin immunostaining.
Treatment
Becker's dystrophy is an incurable disease, and patients receive primarily symptomatic and metabolic therapy. However, scientists are actively searching for effective treatment methods, including genetic and cellular ones. Currently, one can only rely on maintaining the patient's motor ability and independence. Medications that can slow the progression of muscular dystrophy:
- actoprotectors (Ethyltheobenzimidazole). Stimulate physical performance and prevent fatigue;
- cholinesterase inhibitors (Neostigmine). Promote neuromuscular conduction, increase smooth muscle tone;
- adenosine triphosphoric acid, or ATP for short. It is used to improve muscle trophism by intramuscular injection 1-2 times a day. The course of treatment is 30-40 injections, after a month's break the course is repeated if necessary;
- anabolic steroids (Methyladrostenediol). The use of drugs in this group helps accelerate the development and renewal of cells, tissues and muscles;
- heart medications;
- vitamins B and E.
Drug therapy for myopathy is combined with physiotherapy, massage and exercise therapy. A set of measures is drawn up taking into account feasible physical stress on the patient in order to avoid excessive overstrain of weakened muscles. In certain cases, patients need to consult an orthopedist who will help select special corrective devices - corsets, shoes, etc.
Manual therapy is one of the ways to correct the patient’s condition
Surgical intervention is performed in cases where the patient experiences too much pain in the muscle tendons - the operation helps to lengthen them by correcting contractures.
Symptoms of muscular dystrophy
- Problems with muscle function
: Often, a family member notices that something is wrong with the child.
Boys with Duchenne muscular dystorphia
begin to walk later than their peers.
Their calf muscles are enlarged; they have difficulty running, jumping, and climbing stairs. They fall frequently and may develop a tendency to walk on their toes. In addition, they have a speech delay. One of the classic symptoms of Duchenne muscular dystorphia
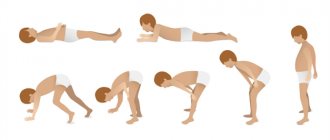
is the so-called positive Gowers test, which manifests itself in the fact that in order to move from a lying position to a standing position, the boy is forced to move his hands and elbows from bottom to top of the body. He is forced to do this due to weakness in his hip muscles. - HIGH CONTENT OF CREATINE KINASE
(CK) IN MUSCLE PROTEIN DETECTED BY BLOOD ANALYSIS.
Detection of a high level of creatine kinase should serve as a signal and urgently contact a specialist to confirm the diagnosis. A high CK indicator is observed in people suffering from other types of muscle diseases, and therefore, to confirm Duchenne muscular dystorphis,
the creatine kinase indicator alone is not enough. - HIGH CONTENT OF SO-CALLED “LIVER” ENZYMES
(AST AND ALT), DETECTED BY BLOOD ANALYSIS. Their high content in the blood is often associated with liver diseases, but it can also be caused by muscular dystrophy. If high concentrations of these enzymes are unexpectedly detected without any other reason, you should be wary. The level of reatin kinase may also be high, and therefore the suspicion of a diagnosis of muscular dystrophy is not without reason. - DELAY OF SPEECH DEVELOPMENT. Children suffering from Duchenne muscular dystorphia
often experience some delay in speech development, which can also be noted
Classification
Nutritional dystrophy has code E41 according to ICD 10.
In gastroenterology, diseases are usually divided according to severity and forms. According to their form, dry (cachectic) and edematous dystrophy are distinguished. In the cachectic form, the disease has a more dangerous course. The edematous form is characterized by widespread edema, including internal edema (pleurisy, pericarditis and ascites), this form is easier to treat.
There are also 3 stages of disease severity:
- The first stage
is characterized by a slight loss of weight and preservation of working capacity with complaints of increased appetite, thirst and frequent urination, weakness and chilliness. - The second stage
is characterized by significant weight loss with decreased performance. Patients with the disease at this stage are still able to care for themselves, but are practically unable to work. Swelling is possible, there is a significant decrease in protein levels, and the level of glucose in the blood often decreases. - The third stage
is characterized by severe exhaustion and the patient’s inability to move independently.
Genetic analysis.
Carrying out a genetic test
necessary in any case, even if the diagnosis of
Duchenne muscular dystorphia
is confirmed by the results of a muscle biopsy. Various types of genetic analysis can provide specific and more detailed information about changes (mutations) in DNA. Having genetic confirmation of the diagnosis is important for several reasons: it can determine whether the boy is eligible to participate in a number of clinical trials regarding a particular mutation, and it also helps the family make decisions related to prenatal diagnosis and future pregnancies. Currently, more than 900 types of mutations are known, which is why accurate diagnosis of dystrophy in Israel is so important, even if you did this test in your homeland.
As soon as the exact mutation or change in the DNA of the dystrophin gene became known to mothers
should be given the opportunity to undergo genetic testing to find out whether they are carriers of the defective gene or not.
This information will be important for maternal relatives (sisters, daughters, aunts, cousins), since it will make it possible to find out whether they themselves are carriers of the defective gene. A genetic diagnosis explained by a genetic
counselor will help the family better understand the test result and the potential impact it may have on everyone in the family.
Diagnostics
The diagnosis can be made based on examination of the patient and history taking. If the anamnesis contains indications of prolonged fasting, there are characteristic symptoms and indicators of laboratory tests (detailed biochemical and clinical blood tests, signs of organ dystrophy are observed on MRI, ultrasound, CT), and other diseases are excluded, then a diagnosis of nutritional dystrophy is made.
The disease should be differentiated from other pathologies that lead to exhaustion: oncological processes (intestinal cancer, stomach cancer and other pathological conditions), diabetes mellitus, tuberculosis, thyrotoxicosis and pituitary disorders.
Nutritional dystrophy differs from other diseases by a pronounced increase in appetite and thirst, hunger, skin changes and severe muscle wasting, bradycardia and a decrease in body temperature, as well as disruption of the endocrine glands.
Muscle biopsy.
Your doctor may recommend a muscle biopsy (that is, a small sample of muscle taken for analysis). The presence of a genetic mutation in Duchenne muscular dystorphia
means that the body either cannot produce the dystrophin protein, or produces it, but in insufficient quantities.
By analyzing a muscle biopsy, you can find out how much dystrophin is in muscle cells. If confirmation of the diagnosis has already been obtained through genetic analysis, a muscle biopsy is not necessary
.
A biopsy does not cancel a genetic test
!
When examining muscle biopsy data, two types of tests are usually performed: immunohistochemical
and
immunoblotting analysis
(method for studying protein antigens) for dystrophin.
These tests determine the presence or absence of dystrophin in a quantitative form; with their help it is possible to distinguish Duchenne muscular dystorphy
from the milder form of muscular dystrophy BMD.
Causes
The cause of nutritional dystrophy is prolonged fasting, when the human body receives an insufficient amount of essential nutrients, and their relative insufficiency is taken into account: if the intake of calories differs from their expenditure.
Starvation is possible for various reasons (environmental disaster war, and other cases of forced long-term lack of food; diets; scars and narrowing of the esophagus, etc.), but the process is aggravated by hypothermia and heavy physical labor.
It should be noted that the development of nutritional dystrophy is possible only in the case of prolonged energy starvation. In this case, the body first depletes the reserves of fat and glycogen, then the reserves of interstitial protein are used to ensure metabolic processes. Dystrophy processes primarily occur in the skin and muscles, after which the disease spreads to the internal organs, and lastly to the vital organs (brain, kidneys and heart).
In the final stages, reserves of minerals and vitamins are depleted, and the immune system ceases to function. Death usually occurs as a result of heart failure or associated infection after suppression of the immune system.