What it is
Irritation of the cerebral cortex is a pathological condition that manifests itself in the form of spontaneous formation of a focus of irritation and excitation in a certain area of the cerebral cortex. Symptoms of irritation are determined by the localization of the pathological condition.
It is necessary to distinguish between normal irritation - irritation of nerve fibers in response to an external stimulus and the formation of an adequate response. For example, when the eyes are exposed to bright light, the pupil contracts (reduces the flow of photons) as a result of irritation of the optic nerve. Pathological irritation is a spontaneous irritation that has no obvious origin and leads to a deterioration in the patient’s quality of life.
Irritation is not included in the list of independent diseases; it is not in the International Classification of Diseases, 10th revision. Irritation of the cerebral cortex acts as a manifestation of the underlying pathology, for example, a tumor of subcortical structures.
Irritation can be focal, when irritation is present in a separate area of the cortex (in the visual or frontal) and diffuse (the entire cortex is irritated).
Irritation of the cerebral cortex also occurs:
- Asymptomatic - irritation of the cortex may not reach the threshold level and not cause signs of the disease.
- Symptomatic - irritation enters the sensitivity threshold and determines the clinical picture.
Higher cortical functions of humans and their disturbances in local brain lesions
Higher cortical functions of humans and their disturbances in local brain lesions
Table of contents
Until now, we have remained within the framework of characterizing the structure and functional organization of individual areas of the cerebral cortex, using mainly methods of morphological and physiological analysis of individual areas of the cerebral cortex.
Over the past decades, however, a new field of science has developed that allows us to take the first steps towards penetrating the most subtle and intimate mechanisms of brain activity. The point is that as a result of the development of experimental technology, it has become possible to begin a physiological analysis of the work of individual neurons, irritating them with an electric current or removing the potentials that arise in them under various conditions that change the behavior of the animal.
Section 1 was written by O. S. Vinogradova.
There is no doubt that this new branch of science provides significant information about the intimate mechanisms of the functioning of nervous tissue and, over time, will allow us to revise many of the currently established ideas about the functional organization of individual sections of the cerebral cortex.
It should be noted from the outset that almost all of the available data on the functioning of neurons in the cerebral cortex were obtained in rabbits or cats, and only a small part of the studies were carried out on monkeys. This means that these data are only of a very basic nature, and they can only be transferred to humans with great caution. However, despite this, data obtained from research at the neural level are already providing significant material that can serve to clarify the general principles of the functional organization of cortical centers and the main trends in their development.
Visual cortex
Careful studies of the primary visual cortex carried out in the laboratory of Jung (1958, 1961) showed the presence of different types of neurons detected when diffuse light was applied. Jung identified 5 main types: A - neurons that do not respond to light; B - responding with a burst of impulses to turning on the light; C - responding by braking to turning on the light; D - responding to turning off the light; E - responding to turning the light on and off. Type A neurons, according to Jung, make up approximately 50% of all neurons in the visual cortex. According to other authors, the number of neurons that do not respond to diffuse light is even greater. As shown by Hubel (1962), special selection of visual signals allows responses to be obtained from all visual neurons. However, for this it is necessary to use formalized, structured visual signals. Hubel showed that this depends on the special organization of the receptive fields of cells in the visual area. It identifies, first of all, cells with so-called “simple receptive fields”. Unlike neurons of the lateral geniculate body with their concentric fields, these cells have elongated active zones, depending on the size, shape, position and direction of movement of the stimulus. Each such active zone is surrounded by a region that has an antagonistic, inhibitory effect on the reactions caused from the center of this region. That is why diffuse illumination, simultaneously exciting the active center and inhibitory periphery of the receptive field of a neuron, prevents its response. In addition to these neurons, Hubel discovered cells with so-called “complex receptive fields.” These fields are larger and less dependent on the exact shape and size of the stimulus. For some neurons, the active stimulus is an edge, a contour, light on one side and dark on the other. Active stimuli for neurons of this type can be varied over a fairly wide range in magnitude and angle of inclination. However, the overall shape and direction of movement of the stimulus remain significant. As we move away from the projection area of the macula, the neuron fields increase in size. Hubel views these neurons as integrators onto which several cells with “simple fields” converge, giving “an abstraction of direction and shape independent of the exact position of an object in the visual field.”
Thus, the cortical projection of the retina (field 17), according to neural studies, is no longer a simple mosaic of topographical points (“point-by-point”), but is an organ of high-order integration, specially adapted for object vision.
Interestingly, when the electrode is moved strictly perpendicular to the surface of the cortex, it is found that all neurons lying under each other have similar receptive fields. This puts forward the principle of organizing the cortical zones of the analyzers into “functional columns”; We will encounter this principle further when considering the organization of other areas.
The described neurons do not exhaust the functional mosaic of the visual area. Neurons were found here that respond only to monochromatic light sources in a narrow spectral band (60-100 m\x) (Andersen, Buchmann, Lennox, 1962). These neurons, which make up approximately 73 of all elements, do not respond to white light, and their response is independent of brightness. They highlight the quality of light over a wide range, regardless of other characteristics of the stimulus. Along with this, Berne (1964) showed that some cells do not change activity with various changes in signal properties, but note all shifts in their intensity.
Many elements of the primary visual area respond to vestibular stimulation (Jung, 1962). In these cases, there is a convergence of visual and vestibular influences on the same neuron, and vestibular influences can significantly modulate responses to visual signals. Therefore, as Jung points out, there is not only the reception of discrete forms of visual information, but also its integration with vestibular and optokinetic stimuli. At the same time, a very small number (no more than 5%) of neurons detecting binocular convergence were found in the primary visual cortex. If convergence was detected, responses arose upon local stimulation of homotopic areas of the retina of two eyes. In this case, the influences were usually antagonistic: for example, excitation from the contralateral eye and inhibition from the homolateral eye. True summation of binocular stimulation has been observed so rarely that most researchers are inclined to conclude that the mechanisms of binocular vision are provided, at least not in the primary visual cortex.
Finally, one more fact should be pointed out, which has been observed many times by different researchers. A fairly large number of neurons in the primary visual area respond to sound, tactile, olfactory and pain stimuli. These responses are more variable than responses to light, have longer latencies, and often converge on light-responsive neurons. Their functional significance is not entirely clear; one can only say that most of them are not associated with the orienting reflex and attention, since they retain their work without a tendency to weaken with prolonged use of the signal.
Auditory cortex
In the primary auditory zone of the cat, the presence of a tonotopic organization is noted at the neural level, although the data of different authors on this issue are not entirely consistent with each other. Katsuki (1962) notes that in the anterior part of the ectosylvian gyrus there are predominantly cells that respond to high tones, in the middle - to mid-frequency tones, and in the posterior - to low tones. Other authors (Evans, Ross, Whitefield, 1965) believe, on the basis of their data, that cells responding to high tones (more than 10 kHz) are indeed located in the anterior part of the Al zone, but cells responding to lower sounds are evenly distributed throughout auditory cortex, so that true tonotopic organization is absent. In any case, one thing is certain: at the cellular level, such an organization turns out to be much more complex, since the neurons of the auditory cortex are characterized not just by certain sound-pitch parameters, but by much more complex functional characteristics, similar to what was shown above for the neurons of the visual cortex.
The proposed classifications of auditory neurons are very complex and varied (Oonishi, Katsuki, 19G5), so we will limit ourselves to a description of some types of responses observed mainly in the auditory cortex of the cat.
Most neurons in area Al respond to applied sounds (short or long) with brief phasic firing responses. Less commonly observed are neurons with a tonic type of response, reacting with a prolonged increase or decrease in the level of activity during the entire duration of the sound. A number of neurons have a narrow optimal frequency1, do not respond to other adjacent frequencies, or respond to them much weaker and at significantly higher thresholds. Galambos and others, for example, described a neuron in the auditory cortex of the cat that responded to a tone of 235 Hz (but not to 234 or 236 Hz), which indicates the extreme subtlety of auditory analysis. However, the most typical for the auditory cortex are cells with very wide bands of optimal frequencies, significantly exceeding in width the parameters characteristic of cells of the medial geniculate body. This, according to Katsuki, suggests that the full pitch analysis, in all likelihood, ends at the thalamic level, and the cortical neurons of the projection area already serve as integrators, onto which several elements of the medial geniculate body converge. This is also confirmed by the fact that cells with several peaks on the reactivity curve are found in the cortex, that is, with several optimal frequencies in different parts of the sound-frequency scale, which is never observed at the lower levels of the auditory analyzer. It is significant that in cortical neurons Katsuki did not observe the dependence of response parameters on sound intensity, which is described by an S-shaped curve, characteristic of the entire auditory system. Only very sharp and strong changes in sound can affect the responses of cortical neurons (usually suppressing them), while changes in intensity within the average range do not affect the responses of neurons. Consequently, the intensity function is encoded by cells at lower levels, and cortical neurons deal with the selection of more informative and subtle characteristics of sound.
The reactions of neurons in the auditory cortex are significantly enhanced when two different sounds are applied simultaneously. In this case, especially clear responses are observed if the sounds are in a harmonic ratio (i.e., their frequencies are related as 1:2, 1:3, etc.) and form beats. In these cases, short phasic responses to the switching on of the current turn into long-term rhythmic reactions. Katsuki associates this phenomenon with the perception at the cortical level of complex timbre characteristics of sound. 10% of neurons in the auditory cortex respond only to frequency-modulated tones. Moreover, many of them are frequency-oriented, that is, they respond only when the tone frequency increases or only when the tone decreases.
1 “Optimal frequency” is a concept corresponding to the “receptive field” in the visual and somesthetic analyzers - the frequency that causes the maximum neuron response at the lowest threshold.
Of course, in the field of hearing it is even more difficult to draw conclusions from data obtained in animals, but the facts described allow us to believe that individual neural elements, which already in a cat can distinguish such diverse and complex characteristics of sound, in humans can be further differentiated for the analysis of individual phonemes and complex acoustic parameters of speech sounds. Galambos et al. (1958) described in the primary auditory cortex of the cat a group of neurons (about 10%) that responded only to complex significant sounds (calling, meowing, mouse squeak). True, these data can be interpreted in a variety of ways - not necessarily as a result of specific cortical integration, but as a consequence of the convergence of influences from activating and “emotional” systems (nonspecific thalamus, limbic formations) on these neurons.
According to Katsuki, the main neurons of the auditory cortex with their fairly wide frequency characteristics are characterized by an anatomical organization into vertical columns with the same functional properties. Individual elements responding to stimuli of non-sound modalities were also found here.
Somatosensory cortex
The posterior central cortex in the area corresponding to the somatotopic projection of somatic sensitivity was studied in cats and monkeys by Mountcastle et al. (1957, 1959, 1966). This area is mainly occupied by elements with local receptive fields and a fairly clear somatotopic arrangement. Receptive fields can sometimes be very limited (2-8 cm2 in the periphery), but, as a rule, receptive fields in the cortex are 15-100 times larger than the fields of somatic first-order afferent neurons. The vast majority of elements of this cortical region respond only to stimulation of the contralateral half of the body. The excitatory receptive field of such neurons is surrounded by a zone, often of a rather complex and irregular configuration, from which it is possible to suppress the cell's response to stimulation of the center of the receptive field. In the area lying anterior to the somatosensory zone, at the transition to the motor cortex of the anterior part of the hemisphere, the number of neurons with huge diffuse receptive fields gradually increases - the response of these cells can often be evoked from all four limbs or from the entire half of the body, with homolateral and contralateral stimulation converge onto one neuron. The latent periods here are longer, and the fields themselves are more labile than in the core of the somatic analyzer. These neurons can be considered as integrators of the fields of neurons in the nuclear zone.
The characteristics of somatic neurons are not limited, however, to their topical representation. Some cells respond only to stimuli of certain submodalities: touching the skin, pulling hairs, pressure on deep tissues, displacement of a limb in a joint. In this case, stimulation of different modalities can be in a reciprocal relationship, and the response to one submodality (touch) can be suppressed by another stimulation of the same receptive field (pressure). Mountcastle discovered in experiments on monkeys the predominant arrangement of the main submodalities along cytoarchitectonic fields (field 3 - cutaneous sensitivity; field 2 - deep sensitivity; field 1 - transition between them).
However, along with this, there are also neurons on which different types of sensitivity converge, so that one cell can respond to the bending of fingers, pressure on the skin and pulling on hairs.
Cell responses can be phasic or tonic. For example, when a limb rotates in a joint, some cells respond with a short burst of impulses at the moment of rotation, while others maintain an increased level of activity throughout the entire time the limb maintains the changed position. Thus, some elements provide information about movement, while others provide information about maintaining a pose. But even in elements with broad convergence of signals, according to some researchers, different sub-modalities or surface points can be encoded in the distribution of cell discharges (pattern), as a result of which the specificity of the information is partially preserved, despite its extensive convergence.
The principle of vertical organization of the cortex was first demonstrated by Mountcastle using the example of the somatosensory area. He showed a significant similarity of receptive fields in columns of neurons recorded when passing the cortex with a microelectrode oriented perpendicular to its surface. Such functional columns were shown to him not only for topical fields, but also for characteristic submodalities. On this basis, Mountcastle considers the vertical columns as integral functional units of the cortex, carrying out discrete analysis and synthesis of corresponding signals.
Secondary Analyzer Zones
Data regarding non-primary areas of cortical analyzers are much more scarce and less certain than the data presented above about primary projection zones. This is quite understandable, since in some experimental animals (rabbit) these areas themselves are poorly expressed and are defined differently by different experimental physiologists. On the other hand, as we showed above, the study of the primary zones themselves requires the experimenter to carefully select very complex and unique stimuli. When studying the non-projection zones of analyzers, the search for adequate signals becomes even more difficult, and various studies of neurons in these areas leave the impression that the data are to a certain extent simplified due to the inadequacy of the applied signals. In this regard, we will limit ourselves to listing only the general distinctive features of secondary areas that have emerged in the studies of different authors.
In the anterior part of the lateral, suprasylvian, ectosylvian gyri, according to the recording of total evoked potentials, secondary responses to light, sound and skin stimulation are equally recorded. Microelectrode studies in these areas reveal a high percentage of neurons with broad multisensory convergence. Typically, the percentage of neurons responding here is lower than in projection areas, but most neurons respond to 2-3 modalities. At the same time, neural studies show the absence of the unambiguity that appears when recording evoked potentials. For example, in the anterior lateral gyrus of a cat, neurons preferentially respond to sound signals and, to a lesser extent, to light and tactile stimuli, and in the suprasylvian gyrus, responses to light signals, etc. predominate. Thus, each of the areas has its own set of sensory integration and - accordingly - specific functions (Dubner, 1966).
The most in-depth study of the specific sensory functions of non-primary areas of the visual area (Brodmann's areas 18 and 19) was recently carried out by Hubel and Wiesel (1966). According to them, these areas, like the primary zone, have a topographic organization; however, it is possible that the peripheral retina is not represented at all. In fields 18 and 19, a progressive complication of the receptive fields of neurons was found, and the change in functional characteristics exactly coincides with the cytoarchitectonic boundaries of the fields. In field 18 there are almost no simple fields (see above) and 90% are cells with complex fields. Cells with hypercomplex fields (5-10%) also appear here. Lower-order hypercomplex cells respond to visual complexes (“limited moving edge” and “doubly limited moving edge”); To simplify somewhat, we can say that they respond to corners and rectangles, strictly rounded in size, width of the surrounding "bounding edge", orientation and direction of movement. Higher-order hypercomplex cells respond to two groups of visual signals with orientations that differ by 90°. In terms of their properties, their fields are clearly characterized as the result of the synthesis of several complex or hypercomplex fields of a lower order. Such elements make up more than half of the cells of the 19th field. Individual cells of this field have complex fields, and in vertical columns complex and hypercomplex cells with the same field orientation are often found, forming discrete functional systems.
Thus, in fields 18 and 19, at the neural level, the generalized reception of complex visual information predominates, highlighting not the contour (as in field 17), but violations of a continuous contour (breaks in lines, curvature, turns, changes in the direction of movement). In addition, in the 18th field, binocular synthesis of information apparently occurs to a significant extent. The secondary somatic zone has a special specificity, where the known topical organization of the somatosensory representation is preserved, but unlike the primary zone, in this representation both halves of the body (homo- and contralateral) are superimposed on each other. The physiological meaning of this fact is not entirely clear. There are also many neurons with very large receptive fields, often they also respond to sounds (Mountcastle, 1962; Carreras, 1963).
Neurons in the secondary auditory area (A II) often do not have an optimal frequency at all and respond to sound regardless of its physical characteristics (Katsuki, 1962). In the secondary areas of the auditory cortex, a significantly larger number of neurons have tonic-type responses. The reactions occur after a longer latent period, but are maintained throughout the duration of the signal and often persist for some time after its cessation, while the primary is dominated by phasic cell responses to the inclusion of a sound signal.
The responses of neurons in the secondary areas are much more labile; they are variable both in receptive fields and in reproducing the structure of the response (distribution of charges over time). These responses are primarily suppressed when small doses of drugs are administered, which indicates their multisynaptic nature (Carreras and Andersen, 1963).
From the presented data it follows, in any case, that the functions of analyzing the elementary physical characteristics of signals are inherent in the secondary areas to a much lesser extent. On this basis, some researchers attribute to multimodal cells of secondary areas the role of diffuse activators, receiving influences from nonspecific activating systems of the brain and regulating “on the spot” the general level of brain activity (including the organization of orientation and attention). However, it is likely that the apparent “simplification” of the total reactions of these cells is, as we said above, a consequence of primitive, inadequate forms of influence. Analysis of interneuron connections and data on the structural organization of these areas, given in the previous section, allow us to assume that neurons in secondary areas are true integrators, generalizing the primary analyzed information within one or several analyzers.
Motor cortex
Data on neurons of the motor cortex (fields 4-6), according to different authors, are very similar. Responses in this area are usually recorded at a significant depth (more than 1000 μ from the surface), due to which the elements from which recording is made are usually identified with large Betz pyramids in layers 5-6. 3U of all cells respond to somatic stimulation of more than two limbs. Responses to irritation of deep tissues, stretching and pinching of muscles, and flexion of limbs in joints dominate. However, a significant number of neurons also respond to tactile stimulation of the skin. Signals from the vestibular apparatus and cerebellum also converge here. All authors, without exception, note the broad convergence of auditory and visual stimulation (Alb-Fessar, 1964; Buzer, 1Θ61; Sokolova, 1966). The activity of a number of neurons increases significantly during movement. In experiments on non-anesthetized monkeys, it was found that in a number of cases neurons that are clearly included in the reaction during active movement of a limb do not change the background impulse during passive movements of the same limb (Evarts, 1965). In other cases, an increase in activity is observed, anticipating movement and coinciding with the general activation reaction in the cortex.
All this allows us to consider the neurons of the motor cortex as elements of a true “final path” or a general zone of sensorimotor integration. Such universal convergence is not observed in any zone of the posterior hemispheres. Obviously, the final results of the analytical-synthetic work of the posterior sections are output to the motor pyramids to control the voluntary motor reactions of the body.
Unfortunately, data on the functioning of neurons in the premotor and frontal parts of the brain are currently lacking.
Dynamic characteristics of cortical neurons
As most researchers who have studied neurons in the primary sensory areas emphasize, their characteristics are strictly constant. The signal that causes a response from a neuron in the projection area is usually encoded in a certain sequence of discharges, which changes only when certain properties of the signal change. The receptive fields of neurons are also strictly constant. Unfortunately, there is currently no possibility of recording the responses of a single neuron over long periods of time (several days), but recording for 8-10 hours in the vast majority of cases does not reveal changes in the characteristics of the neuron’s responses. Therefore, it is assumed that these characteristics are formed on the basis of the anatomical connections of neurons and in the process of functioning at the initial stages (Hubel and Wiesel, 1963), then fixed as functional units that form the analyzer system.
However, the cortex has always been considered as the basic apparatus of learning, attention, memory and individual experience. Therefore, it is natural for researchers to want to discover the correlates of these dynamic processes in cortical neurons.
A number of studies have shown that neuronal responses change characteristics upon stimulation of the activating reticular formation and nonspecific thalamus (Jung, 1958; Fuster, 1951). At the same time, previously inactive cells begin to respond, the probability of responses increases, and in the neurons of the visual cortex the critical frequency of flicker fusion increases. These phenomena can be considered as analogues of attention mechanisms. It has also been shown that the responses of auditory neurons increase when closely viewing a sound source. In the cat visual cortex, viewing a mouse causes a general increase in firing rate while simultaneously decreasing neuronal responses to a diffuse light flash. In the visual, auditory, somatosensory, and motor areas, neurons (“novelty detectors”) have been found that respond only if the stimulus is new or unusual. When the signal is repeated, the responses of such neurons quickly weaken and disappear completely, but can be restored by changing the current signal in some respect (Hubel, Galambos et al., 1959; Vinogradova and Lindsley, 1963, Murata and Kameda, 1963, etc.) . The activity of these neurons can be considered not only as an analogue of the orienting reflex or sensory attention, but also as a correlate of fixation of the trace of the stimulus in the memory system.
Following initial studies by Jasper et al. (1958), who showed a complex redistribution of activity in neurons of different areas of the cortex in a monkey during the development of a motor conditioned reflex, in recent years many works have appeared reproducing the development of conditioned connections on single neurons. When two stimuli are combined, only one of which (“reinforcing”) causes the initial response, it is possible to obtain a “conditioned” response to a previously inactive stimulus. It is significant that the “conditioned” reaction that appears during the combination does not at all reproduce the response to the “reinforcing” signal, which indicates the complex nature of the emerging integration.
Although at present there are only isolated hints, it can be assumed that the element of dynamic transformations will be more pronounced in the secondary and tertiary formations of the crust. In the primary zones, these processes still seem to be the exception rather than the rule. Thus, the number of “novelty detectors” in the visual and auditory areas is only 4-5%. It seems that in the primary zones functions are structured in such a way as to ensure maximum stability of received and transmitted information, as an “objective” reflection of external influences, largely independent of the attitude, interest, meaning and emotional coloring of the signal. On the basis of this stable reflection, in the future, and geographically - in other areas, secondary processing occurs - selection of information according to individual significance, fixation in memory, etc. It is possible that in some respects such processing occurs in non-primary areas of the cortex.
Currently, there is reason to believe that the most ancient region of the cortex, the hippocampus, is somehow involved in these processes. Studies of neurons in this region have revealed an unusually wide convergence of signals from different sensory modalities. However, all these signals cause peculiar tonic responses of neurons only as long as they have the quality of novelty. Reactions quickly adapt and are restored when any parameters of the stimulus change. Neurons with similar dynamics of responses make up about 80% of all reactive neurons of the hippocampus, which suggests the existence of a special role of the hippocampus in the processes of distinguishing between known and unknown signals, fixation of new signals and suppression of responses to signals already recorded in the past experience of the body (Vinogradova, 1964).
These are brief data on the first steps in creating functional cytobarchitectonics of the cortex.
Causes
Pathological irritation of the cerebral cortex has the following causes:
- Inflammatory diseases of the nervous system: neurosyphilis, herpetic encephalitis, meningitis.
- Complications of major diseases: malaria, rubella, measles, meningococcal encephalitis.
- Circulatory disorders in the brain: atherosclerosis, transient ischemic attack, embolism.
- Violation of intracranial pressure due to a tumor.
- Traumatic brain injuries: concussion, bruise.
- Dislocation syndrome.
- Bad habits.
- Working and living in polluted conditions.
general information
Meningioma is a tumor, most often benign.
The neoplasm grows from cells of the dura mater and is localized in the human skull, namely on the surface of the brain or at the base of the skull. Less commonly, the location of meningioma is the ventricles of the brain or bone tissue. This neoplasm is characterized by slow growth. The pathology can be asymptomatic for a long time.
Symptoms
Signs of cortical irritation are determined by the localization of irritation. Symptoms are directly related to the area of the cortex where focal spontaneous irritation occurs:
- Frontal zone. Accompanied by the occurrence of motor reactions. Muscle contraction depends on the location of stimulation in the precentral frontal gyrus. After irritation of the frontal area, complex motor patterns may appear: the patient will begin to tie his shoelaces in the air.
- Temple area. Simple auditory hallucinations (acoasms) and complex hallucinations appear, accompanied by a voice commenting on the content.
- Occipital zone. Accompanied by simple (photopsia) and complex visual hallucinations. Photopsia are second-long hallucinations: flashes of light, a small spot. Complex hallucinations consist of images, the content of which is determined by the patient’s inner mental life.
- The parietal zone is an area of general sensitivity. Tingling, numbness, and crawling sensations occur in different parts of the body. Irritation in this area is also accompanied by perverted sensations of touch, pain, heat or cold.
Diffuse irritation of the cortex is accompanied by small (petit mal) and large (grand mal) convulsions.
Petite seizures include myoclonic spasms of individual muscles. Muscle contraction is characterized by rhythm and absence of complications. Petit mal also manifests itself as absence seizures – short-term loss of consciousness while maintaining muscle tone throughout the body. After 20-30 seconds of “switching off”, patients come to their senses and continue their work. They don't know that they just came out of consciousness.
Grand mal consists of several successive stages:
- Harbingers. The day before extensive seizures, people feel unwell and have a headache. They don't sleep well.
- Aura. Within 30-40 minutes, patients complain of vague pain in the abdomen, arm or heart.
- Tonic phase. The man loses consciousness and falls. All the muscles of the body contract simultaneously and synchronously. Skin color turns blue, breathing is uneven. Duration – no more than 60 seconds.
- Clonic phase. All muscles of the body contract unevenly, asynchronously, chaotically: each muscle contracts separately. Lasts 1-2 minutes.
In general, the entire grand mal seizure lasts up to 3 minutes. After the last phase, the muscles relax and the patient goes into deep sleep. After waking up, he experiences disorientation and retrograde amnesia (he does not remember what happened before the seizure).
Convexital meningiomas
This type of meningiomas is the most common type of tumor. And it is diagnosed in approximately 50% of the total number of tumors.
The most common location is one in which convexital meningioma of the brain is located under the upper lid of the skull (it is formed by the right and left frontal, temporal, parietal and occipital bones). This location of the tumor is observed in 20% of diagnosed cases.
Diagnosis of the disease
To examine pathology, the magnetic resonance imaging (MRI) method is used, which provides detailed information about the disease. The degree of pathological damage and the size of the tumor formation are clearly visible from different angles. This allows us to collect benign pathological examination material to prescribe adequate treatment.
If it is not possible to do an MRI, there is another type of diagnosis - computed tomography. It allows you to clearly examine the tumor for the presence of hemorrhages and salt deposits in the tissue.
The information obtained using MRI and CT is identical. However, during an MRI, the person is not exposed to radiation. To distinguish meningioma from other similar tumors, MR spectroscopy is used. Using magnetic resonance spectra, it is possible to study the metabolic processes of brain tissue.
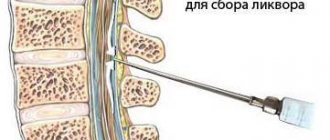
An auxiliary method for diagnosing pathology is electroencephalography. It is used when it is necessary to confirm that epileptic seizures are caused by a tumor. Histological examination is an additional method, used after tumor removal. It allows you to determine whether a tumor is malignant and understand whether it is necessary to continue treatment and prescribe chemotherapy.
Consequences
The prognosis for treatment is most often favorable. But it is worth understanding that and like any disease, convexital meningioma can have a different outcome. The consequences of the disease most often depend on the advanced stage of the disease and the location of the tumor in the human brain. Relapses of the disease are possible. There is a high risk of recurrence of a tumor located in the parietal lobe of the brain.
It is very important to consult a doctor in a timely manner to receive specialized care, since a tumor of the fossa of the skull can lead to complete loss of smell, and a tumor in the area of the sella turcica can contribute to complete loss of vision.
Despite the fact that in most cases, meningioma is a benign formation, this disease can lead to disruption of the auditory, visual and olfactory analyzers, and to disability. If the functioning of vital centers is disrupted, death is possible.
Rehabilitation after treatment
The duration of the rehabilitation period directly depends on the severity of the disease and the location of the tumor in the human brain. In addition, the patient’s recovery rate is affected by the complications caused by the tumor.
In most cases, with visual and hearing impairment caused by atrophy of the corresponding nerves, it is impossible to restore normal operation of the analyzers after surgery.
With atypical and malignant meningiomas, relapses are possible after a course of treatment. However, most often, with timely medical intervention, it is possible not only to get rid of a tumor that causes discomfort, but also to avoid negative consequences and subsequent complications.
Rehabilitation of a patient after a course of treatment aimed at combating meningioma includes several important points:
- the use of acupuncture, which helps to activate nerve endings and restores sensitivity of the limbs after paralysis caused by a tumor;
- conducting drug therapy that maintains the overall tone of the body and prevents relapse;
- physical therapy, thanks to which a person can restore motor and other body functions lost as a result of illness.
For quick and effective recovery after a course of therapy, you must regularly visit your doctor and follow all recommendations.
Treatment
Convexital meningioma can only be treated with surgery. Other types of meningiomas can be dealt with differently.
Currently, there are a huge variety of methods that allow you to influence the tumor without surgical intervention. These methods include:
- stereotactic therapy;
- gamma therapy;
- radiosurgery.
The above treatment methods are not only highly effective, but are also available to a wide segment of the population, since these methods are inexpensive.