Pulse therapy is the administration of high doses of methylprednisolone (most commonly) to quickly suppress the immune response and inflammation. It is used for extremely severe rheumatic diseases (lupus erythematosus, rheumatoid arthritis), acute spinal cord injury, and organ transplantation. Classic regimen: 1000 mg of methylprednisolone (Metypred, Solu-Medrol) intravenously over 60 minutes per 250 ml of saline solution for 3 days in a row.
Complications are most often limited to muscle pain, facial redness, bitterness in the mouth and sleep disturbances. Due to the risk of serious consequences (steroid ulcer, increased blood pressure and sugar, convulsions), pulse therapy is performed only in a hospital. Its effect lasts from 4 to 12 weeks. To restore the body, drugs are prescribed: Panangin, Glyciram, B vitamins, ascorbic acid.
In the meantime, let's talk about Multiple Sclerosis.
The use of the medicine should be discontinued if diagnosed: fungal infection of the skin; inflammation of the cornea of the eye.
Individual intolerance to the drug becomes a ban.
Among the disadvantages of the drug, it is worth highlighting a more significant suppression of the processes of production of corticosteroids by the patient’s adrenal glands, which leads to an increase in the duration of the maintenance course of Prednisolone.
Maintenance therapy for multiple sclerosis is prescribed by the attending physician (neurologist) after extensive medical history collection.
Treatment regimens for MS are tailored individually, depending on the nature of the disease, the severity of accompanying symptoms, etc.
Prevention
Preliminary evidence suggests that people with high levels of circulating vitamin D have a lower risk of MS. Therefore, vitamin D supplementation may reduce the risk of developing MS and converting a first clinical event into a clinically defined form of the disease. Vitamin D may also reduce relapse rates in patients with relapsing-remitting MS.
According to the Institute of Medicine, for healthy people, serum vitamin D concentrations of 20-50 ng/mL are generally considered sufficient for bone and overall health. A serum vitamin D concentration of 30–40 ng/mL has been proposed as optimal for the prevention of MS.
Achieving these levels may require the use of supplemental vitamin D in doses up to 3,000 IU per day. Maintaining these levels requires doses of 500 to 800 IU per day. However, the safety and effectiveness of vitamin D supplementation among patients with MS remains unclear.
Early treatment with immunomodulatory drugs was associated with reduced progression of disability and a reduced incidence of secondary relapses. However, patients with MS should understand that modern immunomodulatory drugs cannot be considered curative.
How does Dexamethasone work?
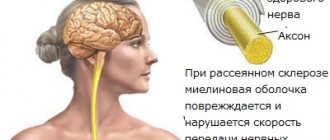
Multiple sclerosis is characterized by a slowdown in the transmission of nerve impulses, which is caused by the destruction of the membrane surrounding the nerve. Taking a corticosteroid prevents the release of lipotropic hormones and ATCG, but at the same time stimulates the release of arachidonic acid into the blood, improving the general well-being of the patient.
The use of Dexamethasone for multiple sclerosis strengthens the body's defenses, as the drug improves the functioning of the cardiovascular system.
Symptoms of exacerbation on average disappear by the end of the first week from the start of using the product.
- blockage of blood vessels by blood clots;
- allergic reaction;
- headache;
- convulsions;
- sleep disorders;
- heart failure;
- increased heart rate;
- skin rashes.
Symptoms of the disease
This treatment method is a good way to get rid of annoying sores, if they really exist. To take the first step towards recovery, pay attention to whether you have the following symptoms:
- Multiple sclerosis disease. In this case, the patient feels a disturbance in coordination of movements, deterioration of vision or speech. The patient feels numbness and constant weakness. There is a feeling of lethargy in the limbs, periodic muscle spasms. All these deteriorations lead to disruption of physical activity, the person begins to stumble.
- Quincke's edema is allergic. Symptoms of such misfortune are visible immediately after a few minutes. Basically, the patient develops swelling in different areas of the mucous membranes and on the face. This is not a simple disease and requires fast and immediate treatment. Remember, if the disease is neglected, the swelling can move to the meninges and cause diseases such as epilepsy.
- Asthmatic status. This is bronchial asthma. In this case, the patient is in an excited state, his breathing becomes difficult, and a paroxysmal cough occurs.
If suddenly you find these symptoms in yourself, you need to visit a doctor. The attending doctor will definitely recommend undergoing a course of treatment with ultra-high and shock doses of hormones. This procedure has beneficial properties and has many advantages.
Metipred pulse therapy usually has a rapid positive effect on articular syndrome in patients with rheumatoid arthritis. Already on days 2-3 from the start of treatment, a significant regression of polyarthritis and morning stiffness is observed, muscle strength and functional activity increase. Unfortunately, the duration of clinical improvement after pulse therapy is not long enough - from several days to 2-3 months. It is for this reason that pulse therapy in patients with rheumatoid arthritis cannot be considered as the main, “basic” means of treatment.
… intravenous administration of ultra-high, loading doses of glucocorticosteroid hormones.
Intravenous administration of ultra-high, shock doses of glucocorticosteroid hormones, or pulse therapy, has become most widespread in the medicine of critical conditions - septic shock, status asthmaticus, acute myocardial infarction with the development of Dressler's syndrome, Quincke's edema, cerebral edema, Lyell's syndrome, multiple sclerosis, etc. The use of pulse therapy for the prevention and relief of a transplant rejection crisis is considered standard. It was the successful use of pulse therapy in patients with a rejection crisis, which is based on a number of immune disorders, that served as the basis for the use of pulse therapy in patients with autoimmune rheumatic diseases.
The pathophysiological rationale for the use of loading doses of glucocorticoids is their ability to actively interact with the immune system and suppress inflammatory reactions. One of the most important effects of pulse therapy is the suppression of the activity of neutrophils and monocytes and the ability to cause transient redistributive lymphopenia. Due to the inhibitory effect of pulse therapy on B-lymphocytes, there is a fairly stable decrease in the production of immunoglobulins with a subsequent decrease in the formation of autoantibodies and CICs (circulating immune complexes).
The anti-inflammatory and immunomodulatory effects of loading doses of glucocorticoids are largely determined by the effect on the cytokine system. Pulse therapy has a pronounced inhibitory effect on the synthesis of anti-inflammatory interleukins-1, -6, -8 (IL) and tumor necrosis factor (TNF), suppresses transcription and enhances the degradation of genes that control the synthesis of IL-2 receptors, which occupy a central place in development immune response, through the influence on the synthesis of lipocortin and metalloproteinases involved in the mechanisms of cartilage destruction, the anti-destructive and anti-inflammatory effects of pulse therapy with glucocorticoids are realized.
Until a certain time, the question of dosages, methods of administration and the drug of choice for pulse therapy remained controversial. In numerous studies of the 80-90s. It has been convincingly proven that intravenous administration of loading doses has the anti-inflammatory and immunosuppressive effects listed above and is significantly more effective than oral administration of glucocorticoids in similar dosages.
The drug of choice so far, of course, is 6-methylprednisolone, which has minimal mineralocorticoid activity and a powerful anti-inflammatory and immunomodulatory effect. Unlike other glucocorticoid drugs, 6-methylprednisolone has balanced genomic and non-genomic effects.
The concentration of methylprednisolone in the blood is proportional to the dose of the administered drug, binding to plasma proteins is linear, 77% is bound to albumin. The duration of action of methylprednisolone is 24-72 hours after infusion; after 24 hours, 99% of the drug is eliminated, and the duration of the effect is ensured by its effect on lymphocyte function. The immunosuppressive and anti-inflammatory activity of pulse therapy depends on the dose, so intravenous administration of 1000 mg of methylprednisolone or more leads to interaction with all glucocorticoid receptors, their reboot and expression, which increases the effectiveness of treatment.
Prednisolone, as a means for pulse therapy, is several times inferior in clinical effectiveness to methylpred (methylprednisolone), does not have such a pronounced immunomodulatory effect and often causes such negative mineralocorticoid effects as fluid retention and arterial hypertension. Dexamethasone is capable of providing a rapid and pronounced antiallergic effect. Its administration may be appropriate in patients with allergic vasculitis and damage to the central nervous system, accompanied by cerebral edema.
The “classical” method of conducting pulse therapy is the intravenous administration of metipred, daily, for 3 consecutive days, at the rate of 15-20 mg per 1 kg of patient weight per day (or 1000 mg per 1 m2 of body surface), which is equivalent to about 1200 mg prednisolone orally. The drug is diluted in 100-250 ml of 0.9% isotonic sodium chloride solution or 5% glucose solution and administered over 35-45 minutes. Slower administration significantly reduces clinical effectiveness, mainly due to a decrease in the immunosuppressive effect. Rapid administration, within 10-15 minutes, can lead to serious complications, including the development of acute heart failure.
A slower administration (about 3 hours) with a dose reduction to 10 mg per 1 kg of weight is advisable in patients with cardiovascular pathology. Only if indicated, heparin (in patients with disseminated intravascular coagulation syndrome, thrombosis), sedatives, panangin and cardiac glycosides are added to the drip. Stimulation with furosemide 15-20 minutes after pulse therapy is allowed only in the case of anuria or oligoanuria.
The combined use of pulse therapy with metipred and cyclophosphamide consists of a classic 3-day pulse therapy with the addition of cyclophosphamide on the 2nd day, at the rate of 15-20 mg/kg of the patient’s weight (or 1000 mg per m2 of body surface). Cyclophosphamide is diluted in one bottle with methylpred in physiological solution or glucose and administered intravenously over 35-45 minutes. To reduce the toxic effects of cyclophosphamide, it is possible to prescribe the patient plenty of fluids. In some cases, 1000 mg of metipred and 1000 mg of cyclophosphamide are prescribed monthly.
Side effects of pulse therapy with methylprednisolone are usually limited to tachycardia and facial hyperemia, which are observed to varying degrees in most patients both immediately during the infusion and several hours later (in rare cases, up to 2 days). In adolescents and people with a predisposition to tachycardia, sedatives are prescribed prophylactically (for example, oxazepam 5-10 mg). Tachycardia is usually easily controlled by the administration of 50 mg of atenolol.
Much less common side effects are emotional agitation (insomnia), bradycardia and hypotension. Prescribing sedatives and hypnotics quickly leads to normalization of emotional status and sleep. Bradycardia and hypotension are observed in no more than 1-2% of cases of pulse therapy and are usually noted during the infusion of methylprednisolone or in the first 1.5-2 hours after its completion. At the first symptoms of hypotension and bradycardia - weakness, dizziness, nausea, blurred vision, it is necessary to immediately begin therapy with cardiotonics; in severe cases, use dexamethasone (16 to 40 mg intravenously).
Among the complications of pulse therapy, the first place is taken by the frequent development of intercurrent infections (bacterial and viral), usually observed in weakened patients, with severe exacerbation of SLE, when pulse therapy is prescribed in combination with cyclophosphamide. there is no real prevention. However, patients should be informed about the possibility of developing infections and take measures to prevent infection. Hiccups are very rarely observed after pulse therapy, but in some cases they can become protracted (from several hours to several days), and can be stopped by subcutaneous administration of atropine. Arthritis of the knee joints, usually the knees, occurs in less than 1% of patients. arthritis, as a rule, develops within a few hours, less than a day, after the infusion, and is stopped by prescribing 100-150 mg of indomethacin or diclofenac.
Anaphylaxis and sudden death are described in the literature as isolated cases. Prevention of anaphylaxis is a thorough collection of an allergic history; if it develops, immediate intravenous administration of 16-40 mg of dexamethasone and norepinephrine is performed. There are several known cases of sudden death of patients receiving pulse therapy. the lethal outcome is observed against the background of developed ventricular arrhythmia, which was probably caused by acute metabolic disorders in the myocardium.
Pulse therapy is not recommended for the development of coronary artery disease, heart failure, severe arrhythmias and uncontrolled arterial hypertension. It is necessary to carry out pulse therapy with particular caution in elderly and senile people. To reduce the risk of cardiac complications, pulse therapy and loop diuretics – furosemide – are prohibited.
Against the background of pulse therapy, in order to prevent ulcerogenic effects, it is necessary to prescribe antacids, H2-histamine receptor blockers, to prevent hypokalemia - potassium preparations, potassium-sparing diuretics, to prevent secondary bacterial complications - antibacterial drugs. Due to the possible development of side effects of pulse therapy (peripheral edema, glycosuria, neuropsychiatric disorders, stomach discomfort, infectious diseases of the skin and genitourinary system, epileptic seizures, blood pressure fluctuations), we recommend its implementation in a hospital setting, under the supervision of a specialist for 3 -7 days, further therapy can be carried out on an outpatient basis. After completing the course of corticosteroids, we recommend taking drugs that stimulate the production of your own steroids by the adrenal glands - glycyram 0.05-0.1 2 to 6 times a day 30 minutes before meals or licorice syrup (or decoction) for 3 to 6 months.
Let's consider the use of pulse therapy for rheumatoid arthritis and multiple sclerosis.
Pulse therapy in patients with rheumatoid arthritis . The main indication for prescribing pulse therapy in patients with rheumatoid arthritis is the development of systemic manifestations or so-called rheumatoid vasculitis. The appearance of persistent febrile syndrome, rheumatoid nodules, weight loss and progressive amyotrophy, widespread lymphadenopathy is a good reason for prescribing “classical” pulse therapy. Three-day pulse therapy with metipred is highly effective in patients with Still's syndrome in adults and Felty's syndrome. Detection of digital vasculitis, severe vascular and trophic disorders (ulcers of the lower extremities, gangrene), polyneuropathy and nephritis in patients with rheumatoid arthritis requires combined pulse therapy with metipred and cyclophosphamide.
Metipred pulse therapy usually has a rapid positive effect on articular syndrome in patients with rheumatoid arthritis. Already on days 2-3 from the start of treatment, a significant regression of polyarthritis and morning stiffness is observed, muscle strength and functional activity increase. Unfortunately, the duration of clinical improvement after pulse therapy is not long enough - from several days to 2-3 months. It is for this reason that pulse therapy in patients with rheumatoid arthritis cannot be considered as the main, “basic” means of treatment.
However, the prescription of pulse therapy in patients with rheumatoid arthritis with predominantly joint damage may be advisable in the case of a progressive course and ineffectiveness of basic drugs and NSAIDs. In patients with a torpid course, rapid progression of joint destruction and ineffectiveness (intolerance) of basic drugs, it seems reasonable to prescribe combination therapy using plasmapheresis, pulse therapy and large doses of methotrexate (20-40 mg) intravenously.
Pulse therapy in patients with multiple sclerosis . The main standard method of treatment for severe exacerbations of multiple sclerosis is the use of regimens of intravenous administration of corticosteroids in pulse doses, which is convincingly confirmed by randomized studies using a double-blind method and the results of dynamic MRI, and the duration of exacerbations and the severity of residual neurological deficit depend on the timely administration of such a course therapy. When prescribing corticosteroids, they primarily rely on a powerful anti-edematous, anti-inflammatory and membrane-stabilizing effect. Therefore, their use is advisable when an exacerbation manifests itself with severe symptoms (lesions of the spinal cord, brain stem, cerebellum) and rapid relief of edema and inflammation can contribute to a more complete regression of symptoms.
MRI is recognized as one of the main methods for making both a diagnosis and criteria for choosing treatment tactics and monitoring treatment. However, in a number of cases, the severity of clinical exacerbation of MS according to MRI data is not confirmed by the appearance of new lesions or an increase in old ones that accumulate contrast, which is apparently associated with decompensation of old lesions and disruption of impulse conduction due to nonspecific changes in homeostasis. The use of corticosteroids in pulse doses in such situations leads to the phenomenon of rapid clinical improvement. Therefore, we believe that in choosing therapeutic tactics for multiple sclerosis, the severity of clinical manifestations should remain the leading one, and MRI data should only be additional.
In the case of mildly expressed signs of exacerbation of multiple sclerosis, but with the identification of “active” foci on MRI that accumulate contrast (Magnevist), or the appearance of additional foci during the study of evoked potentials, is an absolute indication for pulse therapy with a transition after its completion to drugs with neuroprotective effect, and preferably with drugs of prolonged modifying therapy. It is not the number of foci that is fundamental for intensive therapy, but confirmation of the activity of demyelination foci. One demyelinating focus is enough, but it is active and plus a pronounced clinical picture - and this is already the basis for corticosteroid pulse therapy.
Methylprednisolone (metipred, solu-medrol) is administered 500-1000 mg in 200-400 ml of saline solution intravenously, 25-30 drops per minute, 1 time per day in the first half of the day, 3-7 days, depending on the severity of the exacerbation. We recommend using a dose of 1000 mg for patients with severe lesions of the brain stem, ataxia, and vision loss. If disorders of the pyramidal and sensitive areas predominate, the drug is indicated in a daily dose of 500 mg. An increase in disability on the EDSS scale, even in one of the evaluated systems, by more than 1 point, even in the form of a monosymptom (for example, hearing loss, ataxia, facial nerve paresis), requires mandatory pulse therapy.
Solu-Medrol ( Methylprednisolone) is a synthetic analogue of the adrenal hormone (glucocorticoid), which has anti-inflammatory, immunosuppressive (immune suppressive) activity, affects carbohydrate and protein metabolism. For patients with multiple sclerosis, it is prescribed in high doses intravenously to treat severe exacerbations of the disease.
Solu-Medrol ( Methylprednisolone) is a synthetic analogue of the adrenal hormone (glucocorticoid), which has anti-inflammatory, immunosuppressive (immune suppressive) activity, affects carbohydrate and protein metabolism. For patients with multiple sclerosis, it is prescribed in high doses intravenously to treat severe exacerbations of the disease.
Both from the point of view of clinical effectiveness and according to patient reviews, pulse therapy with Solu-Medrol during the treatment of an exacerbation can be called miraculous in most cases. Indeed, in a short period of time, if not complete, then at least a very noticeable restoration of lost functions occurs. However, we will not hide that the pharmacological action of the drug also has a downside: side effects, which sometimes cause very significant inconvenience. Their list may also impress “experienced PC users”.
There is probably no point in listing all the side effects that may manifest themselves during (and after) Solu-Medrol therapy; we will focus on the most common ones: weight gain, acne, osteoporosis and anxiety.
Benefits of using corticosteroids as pulse therapy:
Treatment regimen
The total duration of treatment with Dexamethasone for exacerbation of multiple sclerosis ranges from 10 days to 6 weeks and is carried out in accordance with the developed scheme.
The beginning is always standard and consists of a 5-10-day course of taking high doses of a corticosteroid in the form of injections in combination with taking tablets - this is the so-called pulse therapy or pulse therapy.
How to give Dexamethasone injections for multiple sclerosis? The drug is used according to a specific scheme, intended for slow intravenous, drip or intramuscular (rare) administration. It begins to act 5 – 10 minutes after injection.
The schedule for Dexamethasone injections for multiple sclerosis is given in the protocol for managing patients “Multiple Sclerosis” (approved in 2005 by the Deputy Minister of Health). It looks like this (for 1...8 days the drug is administered intravenously by stream or drip 4 times a day): 1...4 day - 16...40 mg/day; 5...8 day - 8...20 mg/day.
During the period from days 9 to 12, the patient receives Dexamethasone intramuscularly at a dosage of 4...12 mg up to 3 times a day.
Starting from the 13th day, the medicine is either completely discontinued, or a gradual reduction in the dose by 4 mg every other day is practiced.
Studies have shown that the implementation of pulse therapy with Dexamethasone helps to quickly relieve the pathological symptoms of exacerbation of multiple sclerosis.
Reasons for the development of the disease
It is not known exactly why multiple sclerosis develops - the causes of attacks on the myelin sheath of nerves have not been established. It is likely that several factors act together to cause or perpetuate the disease. It has been hypothesized that MS occurs when an environmental agent or event (eg, viral or bacterial infection, chemical exposure, lack of sun exposure) acts in combination with a genetic predisposition to immune dysfunction.
Genetic and molecular factors
First-degree family members (children or siblings) of people with multiple sclerosis are seven times more likely to develop the disorder than the general population.
Different variants of genes commonly found in the general population, usually called polymorphisms, can lead to different gradations in the cellular expression of those genes and, therefore, the proteins they encode. To date, however, only the chromosomal HLA-DRB1 locus has been consistently associated with MS susceptibility. Many other polymorphisms that may act in concert with MS susceptibility have been described using genome-wide approaches, but their individual contribution to risk is not as high as the risk associated with the HLA locus.
Molecular mimicry has been proposed as an etiological process in MS. The molecular mimicry hypothesis refers to the possibility that T cells in the peripheral blood may be activated to attack a foreign antigen and then mistakenly direct their attack to brain proteins that share similar features.
Viral infection
The viral hypothesis is that the virus can infect the immune system by activating myelin-reactive T cells that would otherwise remain quiescent. The virus, which infects cells of the immune and nervous systems, can possibly reactivate periodically and thus lead to acute exacerbations in MS.
Epstein-Barr virus (EBV) infection has been found to reactivate periodically, but a possible causative role in MS is difficult to prove.
Relative evidence of EBV infection as an etiological factor includes:
- long-term studies showing a higher association with MS in individuals with early presence of serum EBV antibodies;
- high expression of EBV antigens in MS plaques.
Evidence that argues against an etiological role for EBV infection includes the fact that MS is a heterogeneous disease. In addition, it is possible that EBV reactivation is only a consequence and not a cause of the disease.
Environmental factors
The incidence of this disease is lower in the equatorial regions of the world than in the southernmost and northernmost regions. However, the latitude gradient began to weaken after 1980, presumably due to the increased incidence of MetS at lower latitudes.
If a person lives in an area with a low incidence of MS before age 15, that person's risk remains low even if they subsequently move to an area with a high incidence. On the other hand, some ethnic groups (eg Eskimos), despite living in areas with higher incidence, do not have a high incidence of MS. Therefore, the exact role of geography and genetics is unclear.
Vitamin D level
Low levels of vitamin D have been proposed as one of the environmental factors contributing to the development of MS. Vitamin D plays a role in regulating the immune response by decreasing the production of pro-inflammatory cytokines and increasing the production of anti-inflammatory cytokines. Additionally, high circulating levels of vitamin D appear to be associated with a reduced risk of multiple sclerosis.
Thus, lower vitamin D levels due to lower exposure to sunlight at higher latitudes may be one cause of geographic variations in the incidence of multiple sclerosis. And the protective effect of traditional diets high in vitamin D may help explain why certain areas (such as Norway) show low incidence of MS despite limited sunlight.
Chronic cerebrospinal venous insufficiency
A controversial hypothesis suggests a vascular rather than immunological cause for some cases of MS. In 2008, Paolo Zamboni described the connection between MS and chronic cerebrospinal venous insufficiency (CCSVI).
The CCSVI hypothesis states that stenosis of the major extracranial venous outflow pathways results in impaired drainage and high levels of cerebral venous reflux. The CCSVI hypothesis has been linked to the potential effects of iron deposition in the brain parenchyma, which some authors have suggested predicts progression of disability, lesion volume accumulation, and atrophy in some MS patients.
Contraindications, possible side effects, overdose symptoms
• slow down the rate of disease progression; • achieve a slowdown in the rate of disability; • reduce the duration, number and severity of exacerbations; • quantitatively reduce foci of demyelination (studies confirmed by the results of magnetic resonance imaging).
1. First line - Avonex, Rebif, Copaxone, Betaferon (beta-interferon derivatives). 2. The second line is fingolimod, tysabri, mitoxantrone. They are more effective compared to the first line, but have a wider range of side effects. The effectiveness of the therapeutic course is achieved within six months.
Alemtuzumab
Study sponsor: Washington Neuropsychology Research Group Co-authors: Genzyme Study titles: CAMMS 223, CAMMS 323 - CARE-MS I, CAMMS 324 - CARE-MS II Development stage: The manufacturing company received a positive decision from the European Medicines Agency to register the drug alemtuzumab (Lemtrada) in July 2013 year. Name of drug: Alemtuzumab (alemtuzumab) Other names: Campas (Campath), Lemtrada (Lemtrada)
Type of MS: Relapsing-remitting multiple sclerosis (MS)
- Alemtuzumab is an investigational monoclonal antibody drug for the treatment of relapsing-remitting multiple sclerosis. It is administered as an intravenous infusion (infusion) for 3-5 days a year.
- Alemtuzumab works by killing T cells, a type of lymphocyte involved in the immune response in MS.
- In a phase III trial, alemtuzumab reduced the number of relapses by approximately 50% compared with interferon beta 1a (Rebif). Some studies have shown that the administration of alemtuzumab in some cases stops the progression of disability, and in some cases the opposite.
- To date, two significant side effects have been identified:
- idiopathic thrombocytopenic purpura (ITP), a disease that interferes with blood clotting. In one case, ITP was fatal.
- overactive thyroid (Graves disease) or thyroid dysfunction
- The manufacturer plans to apply for a license in Europe in the first quarter of 2012
Administration mode
Alemtuzumab is administered intravenously by infusion. In clinical studies, alemtuzumab was administered for 5 days in the first year of treatment, then for 3 days the following year.
Results of the research
The first clinical trials of alemtuzumab included both patients with relapsing and progressive types of MS. In patients with relapsing-remitting MS, treatment with alemtuzumab reduced the frequency of relapses and slowed the progression of disability. A slowdown in the increase in disability was also observed within three years after the end of treatment.
However, less optimistic results were obtained in a clinical trial of 25 people with secondary progressive multiple sclerosis.
MRI scans over a seven-year period showed no new lesions forming in the brains or spinal cords of participants who received alemtuzumab, but disability continued to accumulate in these patients.
This has led researchers to conclude that perhaps something other than inflammation of the myelin sheath is responsible for the progression of MS.
Further studies were aimed at assessing the effectiveness and safety of alemtuzumab for the treatment of patients with relapsing-remitting multiple sclerosis.
Phase 2 Study Results (CAMMS 223):
were published in the New England Journal of Medicine in October 2008. This study included 334 people with active relapsing-remitting MS. The study assessed the effectiveness of two different dosages of alemtuzumab compared with treatment with high-dose interferon beta-1a (Rebif).
The results showed that participants receiving alemtuzumab were significantly less likely to experience disease relapses.
Over three years, 77% of participants who received low-dose alemtuzumab and 84% of participants who received high-dose alemtuzumab did not experience a single clinical exacerbation of the disease, compared with 52% of participants who received interferon beta-1a.
The results also showed that, compared with interferon beta 1a, alemtuzumab reduced the risk of permanent disability by 71%.
Participants in the CAMS 223 study continued to be followed up through Year 4. The results were presented at the Sixty-Second Annual Meeting of the American Academy of Neurology in April 2010.
The data presented showed that 71% of patients who received alemtuzumab were free of disease activity three years after the last course of treatment.
In addition, about 91% of people treated with alemtuzumab did not experience increased disability, compared with 68% of people taking Rebif.
Five years of follow-up of the participants in this study were presented at the 63rd Meeting of the American Academy of Neurology in April 2011.
According to the researchers, 65% of study participants had no clinical disease activity (defined as the absence of relapses and an unsustainable increase in disability measured in points on the Expanded Disability Scale) for four years after the last course of treatment, compared with 27% of patients receiving interferon beta-1a.
However, participants from all groups (i.e., both alemtuzumab and Rebif) whose disease worsened in the first few years were excluded from follow-up.
The results of two large phase III CT studies were published in 2011.
Comparison of Alemtuzumab and Rebif in Multiple Sclerosis Trial I (CARE-MS I) - 323 CAMMS
The purpose of this study was to compare the effectiveness of low dose alemtuzumab with interferon beta-1a (Rebif) for two years.
The study involved 581 patients with relapsing MS who had not previously received preventive therapy for multiple sclerosis. Alemtuzumab reduced relapse rates by 55% compared with interferon beta-1a over two years. Sustained accumulation of disability (as assessed by the EDSS scale) was noted in 8% of participants receiving alemtuzumab and in 11% of participants receiving Rebif.
Comparison of the efficacy of alemtuzumab and Rebif in multiple sclerosis Study II (CARE-MS II) - 324 CAMMS
The second phase III clinical trial compared alemtuzumab with interferon-beta 1a (Rebif) enrolled 840 people who had at least one clinical relapse while on an approved preventive therapy for MS.
In a two-year study, alemtuzumab reduced relapse rates by 49% compared with interferon-1a beta. The researchers noted a 42% reduction in the risk of increased disability, as measured by an increase in EDSS score, in the alemtuzumab group.
The full results of the study are expected to be presented at upcoming major international conferences on MS treatment.
Side effects
The most serious side effects that occurred in patients during treatment with alemtuzumab were:
- Idiopathic thrombocytopenic purpura (ITP), a disease that interferes with blood clotting, was fatal in one case and occurred in 1-3% of participants.
- Thyroid dysfunction was observed in 20-30% of participants.
During the studies, researchers used additional measures to help diagnose and treat these side effects early.
Further research
Comparison of the effects of treatment with Campas (trade name of alemtuzumab) and Rebif on cognitive function in multiple sclerosis (MS)
Participants in the CAMMS 324 study will be tested to examine the effects of alemtuzumab and Rebif treatment on changes in cognitive dysfunction in multiple sclerosis, such as problems with attention, speed of thinking, and memory.
Estimated completion date is end of 2011. More information about this study.
Contraindications, possible side effects, overdose symptoms
- disturbance of consciousness, development of psychoses, hallucinations, disorientation in space;
- increased blood pressure;
- tachycardia;
- increased intracranial pressure;
- nausea ending in vomiting. If you follow medical recommendations, the development of an overdose is completely excluded.
How to use and how long the course of treatment with Dexamethasone will last during exacerbation of multiple sclerosis is decided by the attending physician, since each case is individual.
Personally, I have not used Dexamethasone, it was not prescribed to me, but I have experience using solu-medrol and prednisolone,
I share this in detail in my post HERE
GET WELL
Pulse therapy for multiple sclerosis
Pulse therapy for exacerbation of multiple sclerosis is better known as treatment with hormonal drugs. Common hormones:• Methylprednisolone.• Solu-medrol. Corticosteroids are administered to relieve symptoms during exacerbation (for example, reduce spasticity), slow the progression of the disease in secondary progressive and relapsing forms.
The optimal route of administration is intravenous. The duration of treatment ranges from 3 days to 1 week. The dosage is adjusted individually.
• surges in blood pressure; • infectious diseases; • tuberculosis; • diabetes; • gastrointestinal diseases, accompanied by erosions and ulcers.
Classification
Relapsing-remitting MS (RRMS) - This form of MS is characterized by well-defined acute or subacute attacks with varying degrees of recovery, often with complete or near-complete remission in the early stages. Between attacks the disease is stable. About 85% of initial diagnoses are considered RRMS. More recently, the term "relapsing form of MS" has been used to identify patients with any clinical form of MS that demonstrates clinical relapse.
Secondary progressive MS (SPMS) - begins as RRMS. SPMS is characterized by progressive disability of variable frequency, which may be accompanied by relapses, minor remissions, and plateaus. Of people initially diagnosed with RRMS, 50% or more will develop SPMS within 10 years and 90% within 25 years.
It is important to remember that most of these numbers are based on studies that predate the modification therapy era.
Primary progressive MS (PPMS) —shows a steady rate of progressive disability or occasional plateaus and/or minor remissions from the outset. It is now realized that a small number of these patients may experience clinical relapse. PPMS accounts for about 15% of all MS diagnoses. Clinical isolated syndrome (CIS) is the first episode of a central nervous system neurological event that is typical of multiple sclerosis. Radiologically isolated syndrome (RIS) can be considered "preclinical stage multiple sclerosis". It is defined as lesions typical of MS seen on an MRI scan performed for another reason, such as headache, head injury, or other. Follow-up of many of these patients often demonstrates additional lesions typical of MS and/or a clinical event (CIS) and then RRMS.
Tumor-induced multiple sclerosis is a rare form characterized by tumor-like lesions with signs and symptoms similar to brain tumors. Symptoms are often different from other types of MS and can include headaches, changes in thinking, confusion, speech problems, seizures and weakness. Tumefactive MS often, but not always, develops into RRMS.
Pediatric MS is another rare form of MS. Approximately 3-5% of patients with MS are diagnosed in childhood (under 18 years of age). Diagnosing multiple sclerosis in young children is difficult because the symptoms overlap with many other conditions and may differ from multiple sclerosis in adults.
Manual therapy for multiple sclerosis
1. Acupuncture - targeted insertion of needles under the skin at certain points. This type of alternative treatment has no scientific evidence to support its effectiveness. Expected results: relief of muscle spasms, relief of pain, elimination of tingling or numbness in the extremities, elimination of depression, improvement of bladder function (if there are problems with urination).
2. Massage - effective for back pain, curvature of the spine, cervical osteochondrosis, frequent migraines, muscle tone, excessive fatigue due to constant physical activity or hard work. Massage techniques help improve blood circulation, which has a beneficial effect on the overall well-being of patients.
The most commonly practiced massages are shiatsu, acupressure and Swedish. Under R.S. There are a number of contraindications to massage, so before starting the course you should consult with a leading doctor.
First signs
For many, the first signs of multiple sclerosis appear in what doctors call clinically isolated syndrome (CIS). This episode of neurological symptoms usually lasts 24 hours.
There are two types of CIS:
- Monofocal episode: You have one symptom.
- Multifocal episode: You have more than one symptom.
The most common symptoms in CIS are:
Optic neuritis. Usually only one eye is affected, but in rare cases both can be affected.
Main manifestations:
- blurred vision;
- faded shades;
- pain in the eyes, especially when moving.
Numbness and tingling.
Main manifestations:
- sensation of electric shock when moving the head or neck. it may move along the spine or into the arms or legs;
- numbness in the face;
- tingling in the legs.
Not everyone who has CIS will get MS . The likelihood is higher if there is damage to the brain from loss of myelin.
Bioresonance therapy for multiple sclerosis
This method of restoring the self-regulatory mechanisms of the patient’s body also belongs to the methods of alternative medicine.
The essence of the treatment is to connect the electrodes to a special bioresonance device. The therapy is based on the launch of one's own resources using human electromagnetic oscillations and, in particular, through regular monitoring of the magnetic field.
Thus, pathological vibrations are separated from healthy vibrations, converted into favorable ones, and then returned back to the body.
Wave therapy for multiple sclerosis
Extracorporeal percussion therapy is aimed at treating myofascial syndrome. The impact on certain points occurs due to the pressure generated by a high-energy pulse wave.
Such blows hit the intended target directly, without injuring the tissues that surround the point. ESVP makes it possible to partially restore the function of the damaged spinal cord. Improvements are observed due to the restoration of microcirculation impaired by spinal injuries, pain relief, expansion of capillaries, and improvement of metabolic processes.
Irritations are blocked by changing the polarity of neuronal membranes. The effectiveness of treatment is influenced by the accuracy of determining the location for the wave effect. In combination with drug treatment, the effectiveness of VT increases.
Immune therapy for multiple sclerosis
Immunomodulatory therapy for multiple sclerosis helps fight immunopathological disorders in the body, increasing the duration of remissions.
• Interferon beta - most effective for secondary progressive and relapsing forms of RS. • Rebif - recombinant type human interferon (beta-1a). It is a natural amino acid obtained from cells that are removed from the ovaries of Chinese hamsters.
• Glatiramer acetate.
• hormonal (pulse) therapy; • plasmapheresis; • cytostatics.
Medicines to slow the progression of the disease
Therapy aimed at slowing the progression of the disease is very important and requires high medical qualifications. Since there are many drugs that can specifically act to relieve the disease, it is very important to know which set of drugs will be suitable for each patient.
If the doctor has decided on the direction of therapy, in most cases they begin to use slowing medications such as: ·
- Mitoxantrone in multiple sclerosis is used to stimulate the functional activity of T-suppressors, and the drug can also reduce the secretion of anti-inflammatory cytokines. Research conducted in 2005 confirmed the effectiveness of a drug such as mitoxantrone used for multiple sclerosis. ·
- Neuromidin. This drug allows you to improve and stimulate impulse conduction in the nervous system and neuromuscular transmission. And its main purpose is to improve memory and slow down the progressive course of dementia (dementia).
- Lecithin in multiple sclerosis is used as a component of myelin, which is susceptible to destruction during the course of multiple sclerosis. Experts advise taking this drug together with vitamins B12, since their interaction has the greatest effect from the therapy of this drug.
- An immunomodulatory effect is exerted by a drug such as Interferon beta 1b, the effect of which in multiple sclerosis has been proven by numerous ongoing studies. It is only worth noting that in adult patients with remitting and secondary progressive disease, the use of interferon beta helps reduce the frequency of exacerbations after 2 years by 35%. The severity of relapses is also reduced and the patient’s state of remission is prolonged.·
- IMGs for multiple sclerosis are used to prevent progression of the disease. This medicine for multiple sclerosis is widely recommended, first of all, for its availability, as well as for its effective reduction in the frequency of annual exacerbations.
- Vitamin D in multiple sclerosis is especially important for patients, since many studies show that its deficiency in the body leads to a more severe course of the disease. The recommended daily dose of 600 IU should be adhered to. Maintain the level of this vitamin at the required level.
- Immunoglobulins for multiple sclerosis are used in the treatment of primary progressive and secondary progressive MS diseases. The main positive qualities that immunoglobulins have are ease of use, good tolerability and safety in use. Therefore, today immunoglobulins are widely used in medicine.
Abagio is also used to treat the age group of patients; multiple sclerosis with this medicine can significantly reduce its progression.
Endolymphatic therapy for multiple sclerosis
The treatment regimen is suitable for a recurrent disease, and is based on the administration of drugs directly into the lymph flow of a person suffering from R.S.
A small catheter is inserted into large lymphatic vessels, after which a special dispenser (infusion pump) is connected to the device.
Types of therapy:1. Levin - introduction to tissues.2. Intranodular - lymph node puncture.3. Paranodular - injection of medication into the area adjacent to the lymph nodes.4. Lymphovascular - introduction into the vessel.
Advantages:• increasing the concentrations of drugs in the lymphatic system;• extending the period of action of active pharmacological substances previously introduced into the patient's body;• prompt delivery of the active substance to the brain;• maximizing the anesthetic effect;• inhibiting the destruction of myelin sheaths.
Notes: the list of advantages is based on the results of comparison with the effect of drugs administered intravenously or intramuscularly.
Methods of conducting pulse therapy with metipred
Damage to nerve trunks.
“Post-injection” exacerbation (microcrystalline synovitis).
Uterine bleeding, pancreatitis, increased blood glucose.
Erythema, feeling of heat, sweating, headache.
The concept of steroid arthropathy is based mainly on the results of experimental studies and, as a rule, is associated with too frequent administration of GC (more than once a month).
With careful adherence to asepsis and antisepsis, septic arthritis is very rare (1 case in 46,000 injections).
There is evidence that after the introduction of HA, tendon strength decreases by 40%. Therefore, due to the risk of tendon rupture, it is better not to inject HA into the area of inflamed tendons, especially for athletes. Although domestic authors do not provide data on cases of tendon rupture after the administration of HA for tendonitis.
Other possible complications include soft tissue atrophy, especially common after HA injections into small joints. The formation of calcifications at the site of tissue perforation with a needle has been described.
Sometimes after intra-articular injections the development of so-called post-injection exacerbations is observed. This is manifested by an increase in signs of local inflammation several hours (maximum 48) after administration of the drug. Increased local inflammation may be associated with the crystalline properties of the drug or the effect of preservatives included in the suspension.
Almost all GCs administered intra-articularly also have a systemic effect, which is manifested by a decrease in signs of inflammation in the joints into which GCs were not administered. Other systemic effects are very rarely observed: eosinophilia, lymphopenia, and in patients with diabetes, increased glucose. Uterine bleeding, the development of pancreatitis, and increased blood pressure have been described.
The immediate effect of GC administration may be facial erythema, a feeling of heat, sweating, and headache, which occur in 10% of patients.
SOME RHEUMATIC DISEASES
Systemic use of GCs is one of the most effective methods of pharmacotherapy for a number of rheumatic diseases (Table 13).
In the systemic treatment of GC for rheumatic diseases in the active phase of the disease, 5 main phases are conventionally distinguished:
1. Induction of remission: short-acting GC (prednisolone or methylprednisolone) at a dose of approximately 1 mc/kg/day at 8-hour intervals.
2. Consolidation: switching to a single dose of the entire dose of GC in the morning
3. Dose reduction: the rate of reduction depends on the dose; transition to alternative therapy is possible
4. Maintenance treatment: minimum effective dose of the drug
5. Prevention of complications of GC therapy (starts from the induction phase).
Main indications for systemic use of glucocorticosteroids for rheumatic diseases
Monoclonal therapy for multiple sclerosis
Therapeutic manipulations involve the administration of monoclonal antibodies.
Patented drug names:1. Natalizumab (Tysabri) is a type of integrin alpha-4 antagonist.
Side effects:• increased fatigue;• headaches;• respiratory tract infections;• genitourinary system infections;• rashes;• diarrhea;• anaphylactic reactions.
2. Alemtuzumab (Lemtrada) is a differentiated glycoprotein antibody. A course of treatment initiates the processes of destruction of lymphocytes, followed by cell restoration within 6-12 months. Prescribed for the purpose of monotherapy or in case of contraindications to treatment with Natalizumab.
Innovative drug Alemtuzumab for the treatment of multiple sclerosis
Why is this new medicine needed? An experienced neurologist will be able to answer this question in detail for each patient. Representing a group of antitumor solutions, Alemtuzumab was created on the basis of a monoclonal antibody.
It was developed in the USA (Cambridge University) back in the late 70s of the last century. The drug is prescribed by doctors to effectively influence white blood cells that are affected by cancer and spoil the blood. The medicine is recommended for chronic manifestations of multiple sclerosis and is prescribed in a neurological clinic.
Refers to experimental designs containing monoclonal antibodies. It is used in the treatment of multiple sclerosis, characterized by a relapsing-remitting condition of the patient.
This type of MS (multiple sclerosis) is characterized by wave-like periods of exacerbation. Therefore, when choosing the most effective drugs to reduce inflammation in the central nervous system, one should not forget about innovative developments from this group of drugs.
Pharmacological effects of Campas
According to statistics and scientific publications, the drug in question can significantly reduce the number of relapses. With therapy based on this drug, the disease recedes 75% more often when compared to the effect of conventional drugs based on beta-interferon.
The likelihood of losing ability to work with dosed consumption of Alemtuzumab is ¾ lower compared to traditional methods of treatment.
Patients undergoing treatment with the drug in the system, after 3 years, restore some functions that were previously lost forever.
Patients, according to the results of studies, become more active and healthier from those clients who had to receive traditional therapy (beta-interferon). The latter showed progressive disorders followed by loss of activity.
Information about the clients’ condition is confirmed by scanning the hemispheres, which shows that patients who received doses of Alemtuzumab have an enlarged brain, and the remaining patients are characterized by reduced hemisphere sizes.
Researchers state the fact that systemic use of the drug promotes effective restoration of damaged areas in the brain.
Pharmacokinetic properties
In the international system, this composition is referred to as Alemtuzumab. The initial one-time IV infusion is 75 mg of the active substance. Results can only be achieved from the required dosage of intravenously administered medication.
The concentration of the drug is increased every month of therapy. By the middle of the second month (6 weeks), stable indicators are achieved with an increased concentration of the ingredient in the serum. This helps to reduce lymphocytosis, activated on the basis of tumor processes.
Patients suffering from memory disorders, disorders in which there are more than 30,000 leukocytes per μl in the blood, are characterized by a peak concentration of the drug, pronounced leukocytosis.
In the first 1.5 months. treatment, the effect of the drug is significantly lower when compared with patients whose lymphocyte count does not exceed 30,000/μl.
With an increased concentration of lymphocytes (malignant), a pool of blood cells is formed, where this composition of the drug accumulates.
A significant decrease in lymphocytosis eliminates the pool, while simultaneously increasing the Cmin and Cmax of the administered agent in the serum. The variation in pharmacokinetic data characteristics is confirmed by differences in tumor formations.
Composition of the product
Solutions of the product can be colorless or have a light yellow structure; they are produced in pharmacies as transparent and opalescent substances. The concentrate is available for retail sale. The medicine is used in the preparation of an infusion solution of 1 ml of Alemtuzumab. 10 mg of the drug and 30 mg of additional substances are used.
The composition is presented:
- disodium edetate;
- phosphate buffered saline with components: potassium chloride, potassium dihydrogen phosphate, sodium chloride, dibasic sodium phosphate, polysorbate 80, water (for injection).
Available for sale in ampoules of 3 ml (10 mg/ml), bottles of 1 ml (30 mg/ml) with a package of 3 units.
Mechanism of action
The drug provokes the lysis of lymphocytes by directly interacting with CD52 (antigen), which is not modulated, and can be expressed on B and T lymphocytes, including monocytes, thymocytes and macrophages.
During a lymphocyte crisis, where antibodies have an indirect effect, complement fixation, an antibody-dependent cytotoxic cellular effect, is noted. The specific antigen is found on an insignificant part of the granulocyte (up to 5%) and does not affect platelets or erythrocytes. The medicine spares hematopoietic stem cells and precursors.
Indications and contraindications for use
Alemtuzumab is recommended for use in a dosage of 1 time/week. (iv infusion) for the treatment of multiple sclerosis, with a maximum dosage of 3 mg. The amount is increased in accordance with the prescribed treatment regimen, which lasts for no more than 2 months.
Among the direct restrictions on the use of Campas are:
- for women – the period of feeding the baby;
- hypersensitivity in anaphylactic reactions, manifestations of the drug taken in the system;
- if you are allergic to mouse protein or other substances that are present in the medicine;
- acute form of drug intolerance;
- exacerbations in the systemic process of an infectious chronic disorder;
- presence of HIV;
- malignant formations, tumors;
- state of pregnancy.
Consult your doctor for dosages!
The drug is new and difficult to use, which means the dosage and treatment regimen should be obtained from the attending physician, who will be able to monitor the patient’s condition and correctly prescribe a course of treatment.
It is important to have significant experience in conducting antitumor therapy, then the dosage and method of use of this ingredient will not be difficult to determine.
Much depends on the form of release, as well as the diagnosis of concomitant diseases, the degree of neglect of the identified relapse.
When treating with this medicine, the clinic of elderly patients is carefully monitored.
Overdose and additional instructions
Considering the side effects of the drug, experts state that patients who received a repeat dose up to a volume of 240 mg experienced an overdose. In such situations, record:
- arterial hypotension;
- signs of fever;
- significant anemia (grade III–IV, count according to NCI).
Signs of an overdose may be characterized by fever and allergic reactions of the body. In such situations, the drug is discontinued; no specific antidote has been identified.
Possible analogues (substitutes)
Campas Show all analogues of the drug Lemtrada » Attention: the use of analogues must be agreed with the attending physician.
Active substance, group
Alemtuzumab, monoclonal MIBP antibodies
Dosage form
Concentrate for the preparation of solution for infusion
How to use: dosage and course of treatment
IV infusion, for at least 2 hours.
In the first week of treatment, increasing doses are prescribed - 3 mg on the 1st day, 10 mg on the 2nd day and 30 mg on the 3rd day, provided that each dose is well tolerated. Next - 30 mg per day 3 times a week (every other day). The maximum duration of treatment is 12 weeks.
In most patients, increasing the dose of the drug to 30 mg can be done within 3-7 days.
However, if severe adverse reactions develop (especially decreased blood pressure, chills, fever or bronchospasm) at both a dose of 3 mg and 10 mg, the next daily dose of the drug should be the same and should not be increased until the drug is well tolerated .
Source: //yazdorov.win/nevralgiya/innovatsionnyj-preparat-alemtuzumab-dlya-lecheniya-rasseyannogo-skleroza.html
Pathogenetic therapy for multiple sclerosis
The essence of the method is to prescribe medications that help slow down the development of pathological processes in the central nervous system. This category of drugs includes second- and first-line DMTs.
It has been scientifically proven that even during the period of remission, myelin in the conductors of nerve impulses continues to deteriorate, which negatively affects the functioning of the brain.
Therefore, to maintain and consolidate the therapeutic effect, medications are also taken during periods of relief. The standard dosage regimen is every other day. The dosage is prescribed and adjusted by the treating neurologist.
Definition
Multiple sclerosis (MS) is a chronic neuroimmunological (both nervous and immune systems are affected) autoimmune disorder involving the brain, spinal cord, and peripheral nerves.
Through a mechanism that is not entirely understood, the protective insulating substance called the myelin sheath that covers the nerve is destroyed. The disease gets its name “multiple sclerosis” because the inflammatory attacks, which cause characteristic scars (plaques or spots) of the myelin sheath, manifest themselves unpredictably, vary in intensity, and occur in different parts of the nervous system.
The course of the disease is paroxysmal - periods of exacerbation are followed by periods of remission. The randomness of the location of the next injury can lead to a wide range of neurological symptoms, which can vary from person to person. It has recently become known that the nerve fibers themselves (axons), in addition to the myelin sheaths, are also affected early in the disease process.
Without treatment, the disease leads to permanent disability over several years.