The nervous system of humans and vertebrates has a single structural plan and is represented by a central part - the brain and spinal cord, as well as a peripheral part - nerves extending from the central organs, which are processes of nerve cells - neurons.
Their combination forms nervous tissue, the main functions of which are excitability and conductivity. These properties are explained primarily by the structural features of the membranes of neurons and their processes, consisting of a substance called myelin. In this article we will look at the structure and functions of this compound, and also find out possible ways to restore it.
Why are neurocytes and their processes covered with myelin?
It is no coincidence that dendrites and axons have a protective layer consisting of protein-lipid complexes. The fact is that excitation is a biophysical process, which is based on weak electrical impulses. If electric current flows through a wire, the latter must be covered with insulating material to reduce the dispersion of electrical impulses and prevent a decrease in the current strength. The myelin sheath performs the same functions in the nerve fiber. In addition, it provides support and also provides nutrition to the fiber.
PNS myelin proteins
PNS myelin contains both some unique proteins and several common proteins with CNS myelin proteins.
P0 is the main protein of PNS myelin, has a molecular weight of 30 kDa, and makes up more than half of the PNS myelin proteins. It is interesting to note that although it differs from PLP in amino acid sequence, post-translational modification pathways and structure, both of these proteins are equally important for the formation of the structure of CNS and PNS myelin.
The MBP content in PNS myelin is 5-18% of the total protein, in contrast to the CNS, where its share reaches a third of the total protein. The same four forms of MBP protein with molecular masses of 21, 18.5, 17 and 14 kDa, respectively, found in CNS myelin are also present in the PNS. In adult rodents, MBP with a molecular weight of 14 kDa (named “Pr” according to the classification of peripheral myelin proteins) is the most significant component of all cationic proteins. PNS myelin also contains MBP with a molecular weight of 18 kDa (in this case it is called “P1 protein”). It should be noted that the importance of the MBP family of proteins is not as great for the myelin structure of the PNS as for the CNS.
Chemical composition of myelin
Like most cell membranes, it is of lipoprotein nature. Moreover, the fat content here is very high - up to 75%, and protein - up to 25%. Myelin also contains glycolipids and glycoproteins in small quantities. Its chemical composition differs in the spinal and cranial nerves.
The former have a high content of phospholipids - up to 45%, and the rest is cholesterol and cerebrosides. Demyelination (that is, the replacement of myelin with other substances in nerve processes) leads to severe autoimmune diseases such as multiple sclerosis.
From a chemical point of view, this process will look like this: the myelin sheath of nerve fibers changes its structure, which is manifested primarily in a decrease in the percentage of lipids relative to proteins. Further, the amount of cholesterol decreases and the water content increases. And all this leads to the gradual replacement of myelin containing oligodendrocytes or Schwann cells with macrophages, astrocytes and intercellular fluid.
The result of such biochemical changes will be a sharp decrease in the ability of axons to conduct excitation, up to complete blocking of the passage of nerve impulses.
CNS myelin proteins
The protein composition of CNS myelin is simpler than other brain membranes, and is represented mainly by proteolipids and basic proteins, which make up 60-80% of the total. Glycoproteins are present in much smaller quantities. Myelin of the central nervous system contains unique proteins.
Myelin of the human central nervous system is characterized by a quantitative predominance of two proteins: the positively charged cationic myelin protein (myelin basic protein, MBP) and myelin proteolipid protein, PLP. These proteins are the main components of the myelin of the central nervous system of all mammals.
Myelin proteolipid PLP (proteolipid protein), also known as Folch protein, has the ability to dissolve in organic solvents. The molecular weight of PLP is approximately 30 kDa (Da - Dalton). Its amino acid sequence is extremely conserved, and the molecule forms several domains. The PLP molecule contains three fatty acids, typically palmitic, oleic and stearic, linked to amino acid radicals by an ester bond.
CNS myelin contains slightly smaller amounts of another proteolipid, DM-20, named for its molecular weight (20 kDa). Both DNA analysis and primary structure elucidation showed that DM-20 is formed by the cleavage of 35 amino acid residues from the PLP protein. During development, DM-20 appears earlier than PLP (in some cases even before the appearance of myelin); suggest that in addition to its structural role in myelin formation, it may be involved in oligodendrocyte differentiation.
Contrary to the idea that PLP is required for the formation of compact multilamellar myelin, the process of myelin formation in PLP/DM-20 knockout mice occurs with only minor abnormalities. However, these mice have a reduced lifespan and impaired general mobility. In contrast, naturally occurring mutations in PLP, including normal PLP over-expression, have serious functional consequences. It should be noted that significant amounts of PLP and DM-20 proteins are present in the CNS, the messenger RNA for PLP is also in the PNS, and a small amount of the protein is synthesized there, but is not incorporated into myelin.
Cationic myelin protein (MCP) attracts the attention of researchers due to its antigenic nature - when administered to animals, it causes an autoimmune reaction, the so-called experimental allergic encephalomyelitis, which is a model of a severe neurodegenerative disease - multiple sclerosis.
The amino acid sequence of MBP is highly conserved in many organisms. MBP is located on the cytoplasmic side of myelin membranes. It has a molecular weight of 18.5 kDa and lacks signs of tertiary structure. This core protein exhibits microheterogeneity when electrophoresed under alkaline conditions. Most mammals studied contained varying amounts of MBR isoforms that shared a significant portion of their amino acid sequence. The molecular weight of MBR in mice and rats is 14 kDa. Low molecular weight MBR has the same amino acid sequences on the N- and C-terminal parts of the molecule as the rest of the MBR, but differs in the reduction of about 40 amino acid residues. The ratio of these major proteins changes during development: mature rats and mice have more 14-kDa MBRs than 18-kDa MBRs. The other two isoforms of MBR, also found in many organisms, have molecular masses of 21.5 and 17 kDa, respectively. They are formed by attaching a polypeptide sequence weighing about 3 kDa to the main structure.
Electrophoretic separation of myelin proteins reveals proteins with a higher molecular weight. Their number depends on the type of organism. For example, mice and rats can contain up to 30% of the total amount of such proteins. The content of these proteins also changes depending on the age of the animal: the younger it is, the less myelin there is in its brain, but the more proteins with a higher molecular weight it contains.
The enzyme 2″ 3″-cyclic nucleotide 3″-phosphodiesterase (CNP) makes up several percent of the total myelin protein content in CNS cells. This is much more than in other types of cells. The CNP protein is not the main component of compact myelin; it is concentrated only in certain areas of the myelin sheath associated with the cytoplasm of the oligodendrocyte. The protein is localized in the cytoplasm, but part of it is associated with the membrane cytoskeleton - F-actin and tubulin. The biological function of CNP may be to regulate cytoskeletal structure to accelerate growth and differentiation processes in oligodendrocytes.
Myelin-associated glycoprotein (MAG) is a quantitatively minor component of purified myelin, has a molecular weight of 100 kDa, and is found in the CNS in small quantities (less than 1% of the total protein). MAG has a single transmembrane domain that separates the highly glycosylated extracellular portion of the molecule, composed of five immunoglobulin-like domains, from the intracellular domain. Its overall structure is similar to neuronal cell adhesion protein (NCAM).
MAG is not present in compact, multilamellar myelin, but is found in the periaxonal membranes of oligodendrocytes that form the myelin layers. Let us recall that the periaxonal membrane of the oligodendrocyte is closest to the plasma membrane of the axon, but nevertheless these two membranes do not merge, but are separated by an extracellular cleft. This feature of MAG localization, as well as the fact that this protein belongs to the immunoglobulin superfamily, confirms its participation in the processes of adhesion and information transfer (signaling) between the axolemma and myelin-forming oligodendrocytes during the process of myelination. In addition, MAG is one of the components of the white matter of the central nervous system that inhibits neurite outgrowth in tissue culture.
Among other glycoproteins of white matter and myelin, the minor myelin-oligodendrocytic glycoprotein (MOG) should be noted. MOG is a transmembrane protein containing a single immunoglobulin-like domain. Unlike MAG, which is located in the inner layers of myelin, MOG is localized in its surface layers, which is why it can participate in the transmission of extracellular information to the oligodendrocyte.
Small amounts of characteristic membrane proteins can be identified by polyacrylamide gel electrophoresis (PAGE) (eg, tubulin). High resolution electrophoresis demonstrates the presence of other minor protein bands; they may be associated with the presence of a number of myelin sheath enzymes.
Features of neuroglial cells
As we have already said, the myelin sheath of dendrites and axons is formed by special structures characterized by a low degree of permeability to sodium and calcium ions, and therefore having only resting potentials (they cannot conduct nerve impulses and perform electrical insulating functions).
These structures are called glial cells. These include:
- oligodendrocytes;
- fibrous astrocytes;
- ependymal cells;
- plasmatic astrocytes.
All of them are formed from the outer layer of the embryo - ectoderm and have a common name - macroglia. Glia of the sympathetic, parasympathetic and somatic nerves are represented by Schwann cells (neurolemmocytes).
Structure and functions of oligodendrocytes
They are part of the central nervous system and are macroglial cells. Since myelin is a protein-lipid structure, it helps to increase the speed of excitation. The cells themselves form an electrically insulating layer of nerve endings in the brain and spinal cord, forming already during fetal development. Their processes wrap neurons, as well as dendrites and axons, in the folds of their outer plasmalemma. It turns out that myelin is the main electrical insulating material that delimits the nerve processes of mixed nerves.
Schwann cells and their features
The myelin sheath of the nerves of the peripheral system is formed by neurolemmocytes (Schwann cells). Their distinctive feature is that they are able to form a protective sheath of only one axon, and cannot form processes, as is inherent in oligodendrocytes.
Between the Schwann cells, at a distance of 1-2 mm, there are areas devoid of myelin, the so-called nodes of Ranvier. Along them, electrical impulses are carried out spasmodically within the axon.
Lemmocytes are capable of repairing nerve fibers and also perform a trophic function. As a result of genetic aberrations, the cells of the membrane of lemmocytes begin uncontrolled mitotic division and growth, as a result of which tumors develop in various parts of the nervous system - schwannomas (neurinomas).
Milgamma
Milgamma is a neuroprotector for restoring metabolism inside cells, which allows you to slow down the process of myelin destruction and begin its regeneration. The drug is based on vitamins from group B, namely:
- Thiamine (B1). It is essential for the absorption of sugar in the body and the production of energy. With acute thiamine deficiency, a person's sleep is disturbed and memory deteriorates. He becomes nervous and sometimes depressed, as in depression. In some cases, symptoms of paresthesia are observed (goose bumps, decreased sensitivity and tingling in the fingertips);
- Pyridoxine (B6). This vitamin plays an important role in the production of amino acids, as well as some hormones (dopamine, serotonin, etc.). Despite rare cases of a lack of pyridoxine in the body, due to its deficiency, a decrease in mental abilities and a weakening of the immune defense are possible;
- Cyanocobalomin (B12). It serves to improve the conductivity of nerve fibers, resulting in improved sensitivity, as well as to improve blood synthesis. With a lack of cyanocobalamine, a person develops hallucinations, dementia (dementia), disturbances in heart rhythm and paresthesia are observed.
Thanks to this composition, Milgama is able to stop the oxidation of cells by free radicals (reactive substances), which will affect the restoration of sensitivity of tissues and nerve endings. After a course of taking pills, there is a decrease in symptoms and an improvement in general condition, and the drug must be taken in 2 stages.
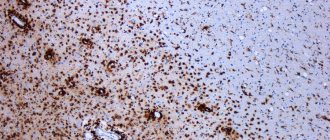
The role of microglia in the destruction of myelin structure
Microglia are macrophages capable of phagocytosis and able to recognize various pathogenic particles - antigens. Thanks to membrane receptors, these glial cells produce enzymes - proteases, as well as cytokines, for example, interleukin 1. It is a mediator of the inflammatory process and immunity.
The myelin sheath, whose functions are to insulate the axial cylinder and improve the conduction of nerve impulses, can be damaged by interleukin. As a result of this, the nerve is “exposed” and the speed of excitation is sharply reduced.
Moreover, cytokines, by activating receptors, provoke excessive transport of calcium ions into the neuron body. Proteases and phospholipases begin to break down the organelles and processes of nerve cells, which leads to apoptosis - the death of this structure.
It is destroyed, breaking up into particles, which are devoured by macrophages. This phenomenon is called excitotoxicity. It causes degeneration of neurons and their endings, leading to diseases such as Alzheimer's disease and Parkinson's disease.
Principles of treatment of shell defects
Diseases associated with the destruction of the pulp membrane are very difficult to treat. Therapy is aimed mainly at relieving symptoms and stopping destruction processes. The earlier the disease is diagnosed, the greater the chances of stopping its progression.
Possibilities of myelin restoration
Thanks to timely treatment, the process of myelin restoration can be started. However, the new myelin sheath will not perform its functions as well. In addition, the disease may enter a chronic stage, and the symptoms will persist, only slightly smoothing out. But even minor remyelination can stop the course of the disease and partially restore lost functions.
Modern drugs aimed at myelin regeneration are more effective, but are very expensive.
Therapy
The following drugs and procedures are used to treat diseases caused by the destruction of the myelin sheath:
- beta interferons (stop the course of the disease, reduce the risk of relapses and disability);
- immunomodulators (affect the activity of the immune system);
- muscle relaxants (help restore motor functions);
- nootropics (restore conductive activity);
- anti-inflammatory (relieve the inflammatory process that caused the destruction of myelin);
- neuroprotectors (prevent damage to brain neurons);
- painkillers and anticonvulsants;
- vitamins and antidepressants;
- cerebrospinal fluid filtration (a procedure aimed at cleansing cerebrospinal fluid).
Pulp nerve fibers
If the processes of neurons - dendrites and axons - are covered with a myelin sheath, then they are called pulpy and innervate skeletal muscles, entering the somatic part of the peripheral nervous system. Unmyelinated fibers form the autonomic nervous system and innervate internal organs.
The pulpal processes have a larger diameter than the non-pulphate ones and are formed as follows: axons bend the plasma membrane of glial cells and form linear mesaxons. They then elongate and Schwann cells wrap repeatedly around the axon, forming concentric layers. The cytoplasm and nucleus of the lemmocyte move to the region of the outer layer, which is called the neurilemma or Schwann membrane.
The inner layer of the lemmocyte consists of layered mesoxon and is called the myelin sheath. Its thickness in different parts of the nerve is not the same.
How to restore the myelin sheath
Considering the role of microglia in the process of demyelination of nerves, we found that under the influence of macrophages and neurotransmitters (for example, interleukins), myelin is destroyed, which in turn leads to a deterioration in the nutrition of neurons and disruption of the transmission of nerve impulses along axons.
This pathology provokes the occurrence of neurodegenerative phenomena: deterioration of cognitive processes, especially memory and thinking, the appearance of impaired coordination of body movements and fine motor skills.
As a result, complete disability of the patient is possible, which occurs as a result of autoimmune diseases. Therefore, the question of how to restore myelin is particularly acute at present. These methods include, first of all, a balanced protein-lipid diet, a healthy lifestyle, and the absence of bad habits. In severe cases of disease, drug treatment is used to restore the number of mature glial cells - oligodendrocytes.
哈尔滨楠木南中医门诊部
source cerebral palsy website - https://help-baby.org/blog/demielinizirjushhie_zabolevanija/2014-02-13-837
Demyelination Demyelination is a disease caused by selective damage to the myelin sheath that runs around nerve fibers.
Demyelination is a pathological process in which myelinated nerve fibers lose their insulating myelin layer. Myelin, phagocytosed by microglia and macrophages, and subsequently by astrocytes, is replaced by fibrous tissue (plaques). Demyelination disrupts the conduction of impulses along the white matter pathways of the brain and spinal cord; peripheral nerves are not affected.
DEMYELINATION - destruction of the myelin sheath of nerve fibers as a result of inflammation, ischemia, trauma, toxic-metabolic or other disorders.
Demyelination is a disease caused by selective damage to the myelin sheath that runs around the nerve fibers of the central or peripheral nervous system. This in turn leads to disruption of the functions of myelinated nerve fibers. Demyelination can be primary (for example, in multiple sclerosis), or develop after a skull injury.
Diseases, one of the main manifestations of which is the destruction of myelin, are one of the most pressing problems of clinical medicine, mainly neurology. In recent years, there has been a clear increase in the number of cases of diseases accompanied by myelin damage.
Myelin is a special type of cell membrane that surrounds the processes of nerve cells, mainly axons, in the central (CNS) and peripheral nervous system (PNS).
The main functions of myelin: • nutrition of the axon • insulation and acceleration of nerve impulses • support • barrier functions.
In terms of its chemical composition, myelin is a lipoprotein membrane consisting of a biomolecular lipid layer located between monomolecular layers of proteins, spirally twisted around the internodal segment of the nerve fiber.
Myelin lipids are represented by phospholipids, glycolipids and steroids. All these lipids are built according to a single plan and necessarily have a hydrophobic component (“tail”) and a hydrophilic group (“head”).
Proteins make up up to 20% of the dry mass of myelin. They come in two types: proteins located on the surface, and proteins immersed in lipid layers or penetrating the membrane through. In total, more than 29 myelin proteins have been described. Myelin basic protein (MBP), proteolipid protein (PLP), and myelin-associated glycoprotin (MAG) account for up to 80% of the protein mass. They perform structural, stabilizing, transport functions, and have pronounced immunogenic and encephalitogenic properties. Among the small myelin proteins, myelin-oligodendrocyte glycoprotein (MOG) and myelin enzymes, which are of great importance in maintaining the structure-function relationships in myelin, deserve special attention.
Myelins in the CNS and PNS differ in their chemical composition ; in the PNS, myelin is synthesized by Schwann cells, with several cells synthesizing myelin for one axon. One Schwann cell forms myelin for only one segment between the areas without myelin (nodes of Ranvier). Myelin in the PNS is noticeably thicker than in the CNS. All peripheral and cranial nerves have such myelin; only short proximal segments of cranial nerves and spinal roots contain CNS myelin. The optic and olfactory nerves contain predominantly central myelin; in the central nervous system, myelin is synthesized by oligodendrocytes, and one cell takes part in the myelination of several fibers.
Myelin destruction is a universal mechanism of response of nervous tissue to damage.
Myelin diseases are divided into two main groups: • myelinopathy - associated with a biochemical defect in the structure of myelin, usually genetically determined
• myelinoclasty - the basis of myelinoclastic (or demyelinating) diseases is the destruction of normally synthesized myelin under the influence of various influences, both external and internal.
The division into these two groups is very arbitrary, since the first clinical manifestations of myelinopathies can be associated with the influence of various external factors, and myelinoclasts most likely develop in predisposed individuals.
The most common disease among the entire group of myelin diseases is multiple sclerosis. It is with this disease that differential diagnosis is most often necessary.
Hereditary myelinopathies
Clinical manifestations of most of these diseases are often observed in childhood. At the same time, there are a number of diseases that can begin at a later age.
Adrenoleukodystrophy (ALD) is associated with insufficiency of the adrenal cortex and is characterized by active diffuse demyelination of various parts of both the central nervous system and the PNS. The main genetic defect in ALD is associated with a locus on the X chromosome - Xq28, the genetic product of which (ALD-P protein) is a peroxisomal membrane protein. The type of inheritance in typical cases is recessive, dependent on sex. Currently, more than 20 mutations in different loci associated with different clinical variants of ALD have been described.
The main metabolic defect in this disease is an increase in the tissue content of long-chain saturated fatty acids (especially C-26), which leads to gross disturbances in the structure and function of myelin. Along with the degenerative process, chronic inflammation in brain tissue associated with increased production of tumor necrosis factor alpha (TNF-a) is of significant importance in the pathogenesis of the disease. The ALD phenotype is determined by the activity of this inflammatory process and is most likely due to both a different set of mutations on the X chromosome and an autosomal modification of the influence of a defective genetic product, i.e. a combination of a basic genetic defect in the sex X chromosome with a peculiar set of genes on other chromosomes.