Methods of using this drug and dosage
Reviews about the drug in question, Ketilept, indicate that it is prescribed only by a doctor. All information provided below is for informational purposes only.
Basically, Ketilept is prescribed to be taken 2 times a day, regardless of food intake. For adults, this medication should be taken according to the following table:
- first day - 50 mg;
- second day - 100 mg;
- third day - 200 mg;
- fourth day - 300 mg.
On the fifth and subsequent days, the daily norm is 300 mg.
The psychiatrist's reviews of the drug "Ketilept" confirm that the clinical response and individual tolerance of each patient are taken into account. Therefore, the dose can be adjusted both up and down, while the daily rate should not exceed 800 mg per day.
In cases where the patient is in stable remission, the attending physician prescribes the lowest dosage. In such cases, patients are periodically examined to determine the need for maintenance therapy with this medication.
If medical therapy was previously completely canceled, and there is a need to urgently resume it, then the daily dose is selected based on the break time.
For example, if only one week has passed since the date of discontinuation, Ketilept continues to be used in the same dosage as before discontinuation of the drug.
If more than one week and one day have passed since the date of cancellation, then the medicine is started according to the initial selection of therapy.
If the patient has a weakened condition or is predisposed to hypertension, then this drug is prescribed at a minimum dose, and the general condition of such a patient must be monitored. In the case when a person has renal or liver failure, this medication is started at 25 mg per day, increasing the dosage daily until the required dose is reached.
KETILEPT
DOSAGE FORM, COMPOSITION AND PACKAGING
White or almost white, film-coated tablets, round, biconvex, engraved with the letter “E” on one side and the number “201” on the other, odorless or almost odorless.
1 tab. quetiapine fumarate 28.78 mg, which corresponds to the content of quetiapine 25 mg
Excipients: microcrystalline cellulose, lactose monohydrate, sodium carboxymethyl starch (type A), povidone, magnesium stearate, colloidal anhydrous silicon dioxide.
Shell composition: hypromellose, titanium dioxide, lactose monohydrate, macrogol 4000, triacetin (triacetylglycerol).
10 pieces. - blisters (3) - cardboard packs. 10 pieces. - blisters (6) - cardboard packs. 30 pcs. - dark glass bottles (1) - cardboard packs. 60 pcs. - dark glass bottles (1) - cardboard packs.
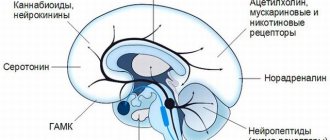
PHARMACHOLOGIC EFFECT
Antipsychotic drug (neuroleptic). Shows higher affinity for serotonin 5-HT2 receptors than for dopamine D1 and D2 receptors in the brain. It also has a higher affinity for histamine and α1-adrenergic receptors and a lower affinity for α2-adrenergic receptors. No significant affinity of quetiapine for m-cholinergic and benzodiazepine receptors was detected.
In standard tests, quetiapine exhibits antipsychotic activity.
Results from studies of extrapyramidal symptoms (EPS) in animals revealed that quetiapine causes mild catalepsy at a dose that effectively blocks dopamine D2 receptors.
Quetiapine causes a selective decrease in the activity of mesolimbic A10 dopaminergic neurons compared to A9 nigrostriatal neurons involved in motor function.
Clinical studies (at a dose of 75-750 mg/day) showed no differences between the use of quetiacin and placebo in the incidence of EPS and the concomitant use of anticholinergic drugs.
Quetiapine does not cause a prolonged increase in plasma prolactin concentrations. Multiple fixed-dose studies have shown no difference in prolactin levels between quetiapine and placebo.
In clinical studies, quetiapine has been shown to be effective in treating both positive and negative symptoms of schizophrenia.
The effect of quetiapine on 5-HT2 and D2 receptors lasts up to 12 hours after taking the drug.
PHARMACOKINETICS
Suction
When administered orally, quetiapine is well absorbed from the gastrointestinal tract. Food intake does not significantly affect the bioavailability of quetiapine.
Distribution
Plasma protein binding is approximately 83%.
Metabolism
Quetiapine is actively metabolized in the liver. It has been established that CYP3A4 is a key enzyme in the CYP-mediated metabolism of quetiapine.
The main metabolites found in plasma do not have pronounced pharmacological activity. Quetiapine and some of its metabolites have weak inhibitory activity against the isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4, but only at concentrations 10-50 times higher than those observed at the usual effective dose of 300-450 mg/day.
Removal
T1/2 is about 7 hours. Approximately 73% of quetiapine is excreted in the urine and 21% in the feces. Less than 5% of quetiapine is not metabolized and is excreted unchanged by the kidneys or feces.
Pharmacokinetics in special clinical situations
The mean plasma clearance of quetiapine is approximately 25% less in patients with severe renal impairment (a CV study of the pharmacokinetics of quetiapine at various dosages when administering quetiapine before or concomitantly with ketoconazole resulted in an average increase in quetiapine Cmax and AUC of 235% and 522 %, respectively, and also led to a decrease in quetiapine clearance by an average of 84%.T1/2 of quetiapine increased, but the average time to reach Cmax did not change.
Based on in vitro results, coadministration of quetiapine with other drugs should not be expected to result in clinically significant inhibition of CYP-mediated metabolism of other drugs.
The average clearance of quetiapine in elderly patients is 30-50% less than in patients aged 18 to 65 years.
The pharmacokinetics of quetiapine is linear; there are no differences in pharmacokinetic parameters between men and women.
INDICATIONS
- acute and chronic psychoses, including schizophrenia;
- treatment of manic episodes in bipolar disorder.
DOSING REGIME
Ketilept should be taken orally 2 times a day, regardless of meals.
In adults, the total daily dose in the first 4 days of therapy is 50 mg (day 1), 100 mg (day 2), 200 mg (day 3) and 300 mg (day 4). Starting from the 4th day, the usual effective daily dose of the drug is 300 mg.
Depending on the clinical response and tolerability of each patient, the dose can be adjusted to range from 150 mg to 750 mg/day. The safety of daily doses above 800 mg has not been assessed in clinical studies.
For acute manic episodes in bipolar disorder, the total daily dose in the first 4 days of therapy is 100 mg (day 1), 200 mg (day 2), 300 mg (day 3) and 400 mg (day 4). ). Further selection of the dose up to 800 mg/day by the 6th day is possible with an increase of no more than 200 mg/day. Depending on the clinical response and tolerability of each patient, the dose can be adjusted to range from 200 mg to 800 mg/day.
The usual effective dose ranges from 400 to 800 mg/day.
To maintain remission, it is advisable to use the lowest dose. Patients should be periodically assessed to determine the need for maintenance therapy.
When resuming therapy less than 1 week after discontinuation of Ketilept, the drug can be continued at the dose used for maintenance therapy. When resuming therapy in patients who have not received Ketilept for more than 1 week, the rules for initial dose selection should be followed and the effective dose should be determined based on the patient’s clinical response.
The recommended starting dose in elderly patients is 25 mg/day, then the dose should be increased by 25-50 mg/day until an effective dose is reached, which is usually lower than in younger patients. Careful selection and reduced doses are also recommended for debilitated patients or those predisposed to hypotensive reactions.
For patients with renal and hepatic impairment, it is recommended to begin therapy with 25 mg/day, then increase the dose daily by 25-50 mg until an effective dose is achieved, depending on the patient's clinical response and individual tolerance.
SIDE EFFECT
The most common side effects of quetiapine are drowsiness, dizziness, dry mouth, mild asthenia, constipation, tachycardia, orthostatic hypotension and dyspepsia. According to summary data from clinical studies, the number of patients who stopped taking the drug due to side effects was approximately the same in the groups receiving placebo and quetiapine. As with other antipsychotics, syncope, neuroleptic malignant syndrome, leukopenia, neutropenia and peripheral edema have been reported during use of quetiapine.
Adverse events observed when taking quetiapine and classified by body system are listed in the following order: very common >1/10); often (1/100); sometimes (1/1000); rarely (On the part of the hematopoietic system: often - leukopenia; sometimes - eosinophilia; very rarely - neutropenia.
From the metabolic side: often - increase in body weight (mainly in the first weeks of treatment), increase in serum transaminases (ALT, AST); sometimes - an increase in the level of GGT, total cholesterol, triglycerides after meals (asymptomatic increases usually disappeared with continued administration of quetiapine); very rarely - hyperglycemia, diabetes mellitus.
From the central nervous system and peripheral nervous system: very often - dizziness, drowsiness (especially during the first two weeks of treatment, usually goes away with continued use of the drug); often - headache, anxiety, psychomotor agitation, tremor, fainting; sometimes - epileptic seizures.
From the cardiovascular system: often - tachycardia, orthostatic hypotension.
From the respiratory system: often - rhinitis, pharyngitis.
From the digestive system: often - dry mouth, constipation, diarrhea, dyspepsia, abdominal pain.
From the reproductive system: rarely - priapism.
Allergic reactions: sometimes - hypersensitivity.
Other: often - mild asthenia, peripheral edema; lower back pain, chest pain, low-grade fever, myalgia, dry skin, decreased visual acuity; rarely - neuroleptic malignant syndrome.
CONTRAINDICATIONS
- pregnancy;
- lactation period (breastfeeding);
- children's age (efficacy and safety have not been established);
- hypersensitivity to the components of the drug.
The drug should be used with caution in patients with liver failure, a history of seizures, cardiovascular and cerebrovascular diseases, or other conditions predisposing to arterial hypotension; in elderly patients.
PREGNANCY AND LACTATION
Category C. The safety and effectiveness of quetiapine during pregnancy has not been established.
The use of Ketilpet during pregnancy is possible only if the expected benefit to the mother outweighs the potential risk to the fetus.
It is unknown whether quetiapine is excreted in breast milk. If it is necessary to use Ketilpet during lactation (breastfeeding), the issue of stopping breastfeeding should be decided.
SPECIAL INSTRUCTIONS
Cardiovascular diseases
Ketilept should be used with caution in patients with known cardiovascular disease, cerebrovascular disease, or other conditions predisposing to hypotension.
Ketilept may cause orthostatic hypotension, especially during the initial period of dose adjustment; it occurs more often in older than younger patients.
There was no relationship between quetiapine and an increase in the QTc interval. However, when prescribing quetiapine simultaneously with drugs that prolong the QTc interval, caution must be exercised, especially in the elderly.
Seizures
There were no differences in the incidence of seizures in patients taking Ketilept or placebo. However, as with other antipsychotic drugs, caution is recommended when treating patients with a history of seizures.
Tardive dyskinesia
Ketilept, like other antipsychotics, can cause tardive dyskinesia with long-term use. If signs and symptoms of tardive dyskinesia occur, dose reduction or discontinuation of the drug should be considered.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome may be associated with antipsychotic treatment. Clinical manifestations of the syndrome include hyperthermia, altered mental status, muscle rigidity, instability of the autonomic nervous system, and increased CPK levels. In such cases, Ketilept should be discontinued and appropriate treatment administered.
Sudden withdrawal reactions
Acute withdrawal symptoms (including nausea, vomiting, insomnia) have been described in very rare cases after abrupt cessation of high-dose antipsychotic drugs. Relapses of symptoms of psychosis and the appearance of disorders associated with involuntary movements (akathisia, dystonia, dyskinesia) are possible. Therefore, if it is necessary to stop taking the drug, a gradual dose reduction is recommended.
Lactose intolerance
When preparing a diet for patients with lactose intolerance, it should be taken into account that film-coated tablets 25 mg, 100 mg, 150 mg, 200 mg and 300 mg contain lactose, respectively, 4.42 mg, 17.05 mg, 25.47 mg, 34.1 mg and 50.94 mg. This drug should not be used in patients with rare hereditary disorders of galactose intolerance, hereditary Sami lactose deficiency or glucose-galactose malabsorption syndrome. Taking into account that quetiapine primarily affects the central nervous system, the drug should be used with caution in combination with other drugs that have a depressant effect on the central nervous system or alcohol.
In controlled clinical studies of quetiapine, there were no cases of sustained severe neutropenia or agranulocytosis. During the observation period after registration of the drug, leukopenia and/or neutropenia resolved after discontinuation of quetiapine administration. Possible risk factors for leukopenia and/or neutropenia include a pre-existing low white blood cell count and a history of drug-induced leukopenia and/or neutropenia.
Like other antipsychotics with alpha1-blocking activity, Ketilept may cause orthostatic hypotension with dizziness, tachycardia and (in some patients) syncope, especially during the initial dose titration period.
In very rare cases, hyperglycemia and worsening of pre-existing diabetes have been observed while taking quetiapine.
A connection has been established between quetiamine intake and a low-dose induced decrease in thyroid hormone levels (T4 and free T4). The maximum reduction occurred during the first two to four weeks of taking quetiapine, but no further reduction occurred with a long course of treatment. Less significant reductions in T3 and reverse T3 were observed only at higher doses of quetiapine. Levels of TSH and TSH (thyroxine-binding globulin) remained unchanged. In almost all cases, discontinuation of quetiapine resulted in restoration of T4 and free T4 levels, regardless of the duration of treatment. No clinically significant hypothyroidism was detected.
Use in pediatrics
The effectiveness and safety of quetiapine in children and adolescents has not been established.
Impact on the ability to drive vehicles and machinery
Due to its effect on the central nervous system, Ketilept may cause drowsiness. Therefore, in the first stages of treatment, for an individually determined period of time, the patient should be prohibited from driving motor vehicles or dangerous machinery. In the future, the degree of restrictions is set individually.
OVERDOSE
Data on overdose of quetiapine are limited.
Symptoms: drowsiness, excessive sedation, tachycardia, decreased blood pressure. Cases of severe overdose of quetiapine leading to death or coma have been reported extremely rarely.
Treatment: There are no specific antidotes to quetiapine. Symptomatic therapy and measures aimed at maintaining respiratory function, the cardiovascular system, ensuring adequate oxygenation and ventilation are carried out.
Medical observation should be continued until the patient recovers completely.
DRUG INTERACTIONS
Particular caution is required when prescribing Ketilept in combination with other drugs that act on the central nervous system.
The results of an in vitro study showed that quetiapine and 9 of its metabolites in vivo are weak inhibitors of metabolic processes mediated by isoenzymes of the cytochrome P450 system (1A2, 2C9, 2C19, 2D6 and 3A4). CYP3A4 is the main enzyme responsible for P450-mediated metabolism of quetiapine.
Effect of other drugs on Ketilept
Phenytoin: simultaneous use of Ketilept with phenytoin leads to an increase in plasma clearance of quetiapine, because Phenytoin induces cytochrome P450 isoenzyme 3A4. The combination of quetiapine (250 mg 3 times/day) and phenytoin (100 mg 2 times/day) increased the average clearance of quetiapine after oral administration by 5 times.
To correct symptoms of schizophrenia in patients receiving concurrent quetiapine and phenytoin, increased doses of Ketilept or other liver enzyme inducers (including carbamazepine, barbiturates, rifampicin, corticosteroids) may be required. In these cases, caution is required when discontinuing phenytoin and/or switching to valproate, which does not have enzyme-inducing properties.
Carbamazepine: Concomitant use of Ketilept with carbamazepine significantly increases the clearance of quetiapine, which leads to a decrease in systemic exposure of quetiapine. Due to this interaction, higher doses of Ketilept may be required.
CYP3A inhibitors: simultaneous use of Ketilept with ketoconazole (200 mg / day for 4 days), a strong inhibitor of the CYP3A isoenzyme, reduces the clearance of quetiapine after oral administration by 84%, resulting in an increase in the concentration of quetiapine in the blood plasma by an average of 235 %. Caution is required when combining Ketilept with ketoconazole and other inhibitors of cytochrome P450 isoenzymes, azole antifungals and macrolide antibiotics (including itraconazole, fluconazole, erythromycin); the dose of quetiapine should be reduced accordingly.
Cimetidine: Regular daily administration of cimetidine (400 mg 3 times daily for 4 days), which is a nonspecific enzyme inhibitor, resulted in a 20% reduction in the mean plasma clearance of quetiapine (150 mg 3 times daily) after oral administration. . When using Ketilept with cimetidine simultaneously, there is no need to change the dose of the former.
Thioridazine: Thioridazine (200 mg 2 times/day) increased the clearance of quetiapine (300 mg 2 times/day) from plasma after oral administration by 65%.
Risperidone and haloperidol: simultaneous use of quetiapine (300 mg 2 times / day) with the antipsychotic drug haloperidol (7.5 mg 2 times / day) or risperidone (3 mg 2 times / day) did not change the equilibrium pharmacokinetics of quetiapine.
Fluoxetine and imipramine: simultaneous use of quetiapine (300 mg 2 times / day) with the antidepressant and CYP3A4 and CYP2D6 inhibitor fluoxetine (60 mg 1 time / day) or the known CYP2D6 inhibitor imipramine (75 mg 2 times / day) did not change the equilibrium pharmacokinetics of quetiapine.
Effect of Ketilept on other drugs
Antipyrine: repeated daily administration of quetiapine (up to 750 mg/day with 3 doses) did not cause clinically significant changes in the clearance of antipyrine or its metabolites. This indicates that quetiapine does not have a significant inhibitory effect on liver enzymes involved in cytochrome P450-mediated metabolism of antipyrine.
Lithium: simultaneous use of quetiapine (250 mg 3 times / day) with lithium did not affect any pharmacokinetic parameters of lithium at steady state.
Lorazepam: The mean oral clearance of lorazepam (single dose 2 mg) was reduced by 20% while taking quetiapine (250 mg three times daily).
Smoking did not affect the clearance of quetiapine from blood plasma.
Since clinical studies have shown that quetiapine potentiates the cognitive and motor effects of alcohol in patients with psychosis, alcohol should not be consumed during treatment with Quetiapine.
CONDITIONS OF DISCHARGE FROM PHARMACIES The drug is dispensed with a prescription.
CONDITIONS AND DURATION OF STORAGE
The drug should be stored out of the reach of children at a temperature not exceeding 25°C.
Shelf life Blister bottle Film-coated tablets 25 mg 2 years 3 months 3 years Film-coated tablets 100 mg 3 years 3 years Film-coated tablets 150 mg 2 years 3 months 3 years Film-coated tablets 200 mg 2 years 6 months 2 years 6 months Film-coated tablets 300 mg 2 years 6 months 2 years 6 months
Do not use after the date indicated on the package.
What side effects may occur after taking it?
Reviews of the drug Ketilept indicate that the most common side effects are the following:
- drowsiness;
- dizziness;
- dry mouth;
- development of asthenia;
- constipation;
- tachycardia;
- development of hypotension.
It is worth noting that the above symptoms appear quite rarely. However, it is strictly forbidden to take this medication on your own. It is important to understand that patients use this medication only under the supervision of the attending physician.
What are the contraindications to the use of this product?
Reviews of the drug "Ketilept" confirm that the use of this drug is not recommended in the following situations:
- during pregnancy;
- during breastfeeding;
- patients under 14 years of age;
- with hypersensitivity to one of the components that make up the medication.
Also, this drug is prescribed with caution to patients who have liver failure and may experience seizures. This drug is not recommended for the elderly, but if there is an urgent need to take it, then use is carried out under the strict supervision of a doctor.
Compound
| Film-coated tablets | 1 table |
| active substance: | |
| quetiapine fumarate | 28.78 mg |
| 115.13 mg | |
| 172.7 mg | |
| 230.26 mg | |
| 345.4 mg | |
| (equivalent to 25 mg, 100 mg, 150 mg, 200 mg and 300 mg quetiapine, respectively) | |
| excipients: MCC - 9.22/36.87/55.3/73.74/110.6 mg; lactose monohydrate - 4/16/24/32/48 mg; sodium carboxymethyl starch (type A) 3.5/14/21/28/42 mg; povidone - 3/12/18/24/36 mg; magnesium stearate - 0.75/3/4.5/6/9 mg; colloidal silicon dioxide anhydrous - 0.75/3/4.5/6/9 mg | |
| shell composition (25 mg tablets): Opadry II33G28523 white (hypromellose - 0.8 mg (40%); lactose monohydrate - 0.42 mg (21%); macrogol 4000 - 0.16 mg (8%); titanium dioxide - 0.5 mg (25%); triacetin - 0.12 mg (6%) | |
| shell composition (100 mg tablets): Opadry II33G28523 white (hypromellose - 2 mg (40%); lactose monohydrate - 1.05 mg (21%); macrogol 4000 - 0.4 mg (8%); titanium dioxide - 1, 25 mg (25%); triacetin - 0.3 mg (6%) | |
| shell composition (150 mg tablets): Opadry II33G28523 white (hypromellose - 2.1 mg (40%); lactose monohydrate - 1.1025 mg (21%); macrogol 4000 - 0.42 mg (8%); titanium dioxide - 1.3125 mg (25%); triacetin - 0.315 mg (6%); Opadry II33G24283 pink (hypromellose - 0.7 mg (40%); iron oxide yellow dye - 0.0105 mg (0.6%); dye iron oxide red - 0.032 mg (1.83%); lactose monohydrate - 0.3675 mg (21%); macrogol 4000 - 0.14 mg (8%); titanium dioxide - 0.3981 mg (22.57%) ; triacetin - 0.105 mg (6%) | |
| shell composition (200 mg tablets): Opadry II33G28523 white (hypromellose - 2 mg (40%); lactose monohydrate - 1.05 mg (21%); macrogol 4000 - 0.4 mg (8%); titanium dioxide - 1, 25 mg (25%); triacetin - 0.3 mg (6%); Opadry II33G24283 pink (hypromellose - 2 mg (40%); yellow iron oxide dye - 0.03 mg (0.6%); iron oxide dye red - 0.0915 mg (1.83%); lactose monohydrate - 1.05 mg (21%); macrogol 4000 - 0.4 mg (8%); titanium dioxide - 1.1285 mg (22.57%) ; triacetin - 0.3 mg (6%) | |
| shell composition (300 mg tablets): Opadry II33G28523 white (hypromellose - 5.6 mg (40%); lactose monohydrate - 2.94 mg (21%); macrogol 4000 - 1.12 mg (8%); titanium dioxide - 3.5 mg (25%); triacetin - 0.84 mg (6%) |
Use during pregnancy and lactation
Reviews from a psychiatrist about the drug Ketilept confirm that to date its safety and effectiveness during pregnancy have not been established. Therefore, in the vast majority of cases, the use of such a drug is not recommended.
But at the same time, in some cases, the appointment of such a drug is still possible, but only in a situation where the benefit to the expectant mother will outweigh the possible risk to the child. As for the period of breastfeeding, it is currently not precisely established whether the active ingredient quetialin enters the milk or not. Therefore, if there is an urgent need to take this drug, breastfeeding should be stopped immediately.
Pharmacodynamics and pharmacokinetics
Quentiax tablets are classified as atypical antipsychotics similar to serotonin . The drug also shows affinity for histamine and alpha-1 receptors and slightly with alpha-2 receptors.
The use of these tablets in the prescribed dosage does not cause extrapyramidal symptoms and does not lead to a long-term increase in the concentration of prolactin in the blood plasma .
The positive therapeutic effect, observed from the very beginning of taking the tablets, is maintained for a long time. Their effect on serotonin and dopamine receptors lasts more than 12 hours.
This drug is characterized by high absorption, while its bioavailability does not depend on food intake. Plasma protein binding reaches about 83%. In the body, the drug undergoes active metabolism in the liver. As a result, pharmacologically inactive metabolites . The drug is excreted in several stages in metabolites and unchanged form through the kidneys and intestines.
Special instructions for the drug "Ketilept"
Application (reviews also confirm this) should be careful for the following diseases:
- heart diseases;
- seizures;
- tardive dyskinesia;
- neuroleptic malignant syndrome;
- sudden withdrawal reaction;
- for lactose intolerance.
All this is confirmed by patient reviews about the drug Ketilept. If a person suffers from heart disease or vascular defects of the brain, then this drug is prescribed with caution and in a minimal dose. The fact is that Ketilept can provoke orthostatic hypotension, which can manifest itself at the initial stage of treatment. As a rule, this problem occurs in elderly people, so they should start taking the drug strictly under the supervision of their doctor.
To date, there have been no cases of a person experiencing seizures after taking this medication. But this drug, as well as other antipsychotic drugs, can provoke such a reaction. Therefore, it is necessary to start taking this medication with extreme caution.
This is also described in detail in the instructions for the drug "Ketilept".
Reviews also indicate that it can provoke tardive dyskinesia if used for a long time. Therefore, if such an unpleasant symptom makes itself felt, you should immediately contact your doctor to adjust the dose taken downward or to completely discontinue the drug.
Pharmacological group
| Category ICD-10 | Synonyms of diseases according to ICD-10 |
| F20 Schizophrenia | Dementia praecox |
| Bleuler's disease | |
| Sluggish schizophrenia | |
| Sluggish schizophrenia with apatoabulic disorders | |
| Exacerbation of schizophrenia | |
| Acute stage of schizophrenia with agitation | |
| Acute form of schizophrenia | |
| Acute schizophrenia | |
| Acute schizophrenic disorder | |
| Acute attack of schizophrenia | |
| Psychosis discordant | |
| Psychosis of schizophrenic type | |
| Dementia early | |
| Febrile form of schizophrenia | |
| Chronic schizophrenia | |
| Chronic schizophrenic disorder | |
| Cerebral organic failure in schizophrenia | |
| Schizophrenic conditions | |
| Schizophrenic psychosis | |
| Schizophrenia | |
| F29 Non-organic psychosis, unspecified | Hallucinatory-delusional disorders |
| Hallucinatory-delusional syndrome | |
| Intoxication psychosis | |
| Manic-delusional disorders | |
| Manic chronic psychoses | |
| Manic psychosis | |
| Acute psychosis | |
| paranoid psychosis | |
| Paranoid psychosis | |
| Subacute psychosis | |
| Presenile psychosis | |
| Psychosis | |
| Intoxication psychosis | |
| Paranoid psychosis | |
| Psychosis in children | |
| Psychoses of childhood | |
| Psychomotor agitation in psychosis | |
| Reactive psychoses | |
| Chronic psychoses | |
| Chronic hallucinatory psychosis | |
| Chronic psychosis | |
| Chronic psychotic disorder | |
| Schizophrenic psychosis | |
| F31.1 Bipolar affective disorder, current episode of mania without psychotic symptoms | Mania in bipolar disorder |
| F31.2 Bipolar affective disorder, current episode of mania with psychotic symptoms | Manic episode of bipolar disorder |
| Mania in bipolar disorder | |
| F31.9 Bipolar affective disorder, unspecified | Depressive episode of bipolar disorder |
| F32 Depressive episode | Adynamic subdepression |
| Astheno-adynamic subdepressive states | |
| Astheno-depressive disorder | |
| Astheno-depressive state | |
| Asthenodepressive disorder | |
| Asthenodepressive state | |
| Major depressive disorder | |
| Sluggish depression with lethargy | |
| Double depression | |
| Depressive pseudodementia | |
| Depressive illness | |
| Depressive mood disorder | |
| Depressive disorder | |
| Depressive mood disorder | |
| Depressive state | |
| Depressive disorders | |
| Depressive syndrome | |
| Depressive syndrome larvated | |
| Depressive syndrome in psychosis | |
| Masked depressions | |
| Depression | |
| Depression of exhaustion | |
| Depression with symptoms of lethargy within the framework of cyclothymia | |
| Depression smiling | |
| Involutional depression | |
| Involutionary melancholy | |
| Involutional depressions | |
| Manic-depressive disorder | |
| Masked depression | |
| Melancholic attack | |
| Neurotic depression | |
| Neurotic depression | |
| Shallow depressions | |
| Organic depression | |
| Organic depressive syndrome | |
| Simple depression | |
| Simple melancholic syndrome | |
| Psychogenic depression | |
| Reactive depression | |
| Reactive depression with moderate psychopathological symptoms | |
| Reactive depressive states | |
| Reactive depression | |
| Recurrent depression | |
| Seasonal depressive syndrome | |
| Senestopathic depression | |
| Senile depression | |
| Senile depression | |
| Symptomatic depression | |
| Somatogenic depression | |
| Cyclothymic depression | |
| Exogenous depression | |
| Endogenous depression | |
| Endogenous depressive states | |
| Endogenous depression | |
| Endogenous depressive syndrome |
Signs of neuroleptic syndrome
Often, during medical therapy, a person is diagnosed with neuroleptic malignant syndrome. It can be identified by the following symptoms:
- hyperthermia;
- changes in mental status;
- the appearance of muscle rigidity;
- instability of the nervous system.
If such signs are detected, then you should immediately stop using Ketilept and, accordingly, carry out medical therapy to eliminate this defect.
If a person experiences a sudden withdrawal reaction, they will experience the following symptoms:
- signs of acute withdrawal;
- severe nausea;
- gagging;
- insomnia.
It is worth emphasizing that such a reaction of the body occurs in very rare cases. It mainly manifests itself if the antipsychotic drug is abruptly stopped. In this case, a mental disorder is added to all the signs described above. In order to avoid this effect, the patient should gradually reduce the dosage of the drug used and only then completely abandon it.
If a person has lactose intolerance, then this medication is replaced with an analogue. The fact is that the shell that covers the tablets is created with the addition of lactose. Therefore, the drug is not recommended for use. But if it is not possible to switch to an analogue, then the use of such tablets is started extremely carefully, monitoring the manifestation of side effects.
Overdose
As the instructions for use indicate for the drug “Ketilept” (reviews also confirm this), to date there have been cases of overdose, which are expressed in the following symptoms:
- decrease in blood pressure;
- increased heart rate;
- drowsiness;
- manifestation of apathy;
- in rare cases, coma may occur.
If this drug is combined with other antipsychotic medications, fainting and swelling of the entire body may occur.
At the moment, there are no special antidotes that can neutralize an overdose. Therefore, therapeutic therapy is aimed at normalizing the activity of the cardiovascular system, as well as restoring respiratory function. For these purposes, the patient is provided with ventilation and oxygenation.
A prerequisite is complete observation of the victim until his complete recovery.
Reviews after using this medication
There are a lot of reviews for the drug "Ketilept". For depression, it is prescribed quite often.
And the overwhelming majority of reviews about this medication are positive. A very good and effective drug, people who started taking this drug got rid of panic attacks that they had suffered from for a long time. And the main advantage of this medication is that there are practically no side effects. The conclusion suggests itself: Ketilept is an effective antipsychotic medication.
Often people come to the appointment who show signs of schizophrenia. In this case, the drug Ketilept is recommended, since its active substance has a sedative effect. As for side effects, with many years of practice in prescribing this drug, such negative effects were extremely rare. Therefore, when diagnosed with schizophrenia, only this medication is clearly prescribed.
What do experts say about this medication?
Reviews from doctors about the drug “Ketilept” confirm that when prescribing this drug, the patient has to get used to it for a very long time, but the result of such torment is worth it. After using the medicine, all mood swings disappear. And most importantly, apathy towards the world around us disappears.
Initially, the doctor prescribes taking this medication 300 mg per day. As soon as the course of treatment begins, the patient experiences lethargy and constant drowsiness. But gradually these side effects go away, and in return a long-awaited feeling of calm and self-confidence appears.
Release form
Film-coated tablets, 25, 100, 150, 200, 300 mg. 30 or 60 tablets each. in a brown glass bottle with a PE cap with tamper evident and an accordion shock absorber, 1 fl. in a cardboard box.
Ketilept is produced in the form of white, film-coated tablets containing the active substance (quetiapine) in the amount of:
- 100 mg – with engraving “202”;
- 150 mg – with engraving “203”;
- 200 mg – with engraving “204”;
- 300 mg – with engraving “204”.
10 pieces in a blister.
The following drugs are analogues of Ketilept:
- According to the active component - Gedonin, Ketiap, Seroquel, Victoel, Laquel, Nantarid, Quentiax, Servitel, Kutipin;
- By mode of action - Azaleptin, Zalasta, Clozasten, Olanzapine, Leponex, Olanzapin-Teva, Olanex, Saphris, Azaleprol, Zyprexa Adera, Normiton.