| Olanzapine | |
| Olanzapinum | |
| Olanzapine | |
| Chemical compound | |
| IUPAC | 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3- b ][1,5]benzodiazepine |
| Gross formula | C₁₇H₂₀N₄S |
| CAS | 132539-06-1 |
| PubChem | 4585 |
| DrugBank | 00334 |
| Classification | |
| Pharmacol. group | Atypical antipsychotics |
| ATX | N05AH03 |
| Dosage forms | |
| tablets (2.5, 5, 7.5, 10, 15 and 20) | |
| Other names | |
| Olanzapine, Normiton, Olanex, Parnasan, Zalasta, Zyprexa, Egolanza | |
| Olanzapine at Wikimedia Commons | |
Olanzapine
is an antipsychotic drug (atypical antipsychotic), structurally and in action similar to clozapine[1]. Used to treat schizophrenia and bipolar affective disorder. It has a wide range of psychopharmacological effects and has an antidepressant effect [2].
Also produced in combination with fluoxetine under the name Symbiax (English)Russian. for the treatment of bipolar depressive episodes and treatment-resistant depression.
Content
- 1. History
- 2 Pharmacodynamics
- 3 Pharmacokinetics
- 4 Indications
- 5 Dosage regimen
- 6 Side effects 6.1 Metabolic and endocrine disorders
- 6.2 Animal studies
- 8.1 Pediatric use
Pharmacodynamics
Zyprexa (olanzapine), tablets 10 mg
Preclinical studies have established the affinity of olanzapine for serotonin 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, dopamine D1, D2, D3, D4 and D5, muscarinic (1..5) , adrenergic α1 and histamine H1 receptors. Experimental studies have revealed the presence of olanzapine antagonism towards serotonin receptors, dopamine and cholinergic receptors. In vivo
and
in vitro
, olanzapine has a more pronounced affinity and activity for 5-HT2 compared to D2 receptors.
According to electrophysiological studies, olanzapine selectively reduces the excitability of mesolimbic dopaminergic neurons, and at the same time has a slight effect on striatal nerve pathways involved in the regulation of motor functions. Olanzapine reduces the conditioned defense response (a test that characterizes antipsychotic activity) at doses lower than doses that cause catalepsy (a disorder reflecting side effects on motor function). Unlike “typical” antipsychotics, olanzapine enhances the anti-anxiety effect during the anxiolytic test. [ source not specified 2106 days
]
Two placebo-controlled and two of three comparative controlled studies involving 2900 patients with schizophrenia showed that olanzapine provides a statistically significant reduction in the short term of both productive (including delusions, hallucinations) and negative disorders [source not specified 2577 days
]. Data on the effects of different doses of olanzapine on negative symptoms are not entirely consistent; It is possible that the reduction in negative distress may be due to olanzapine's effect on secondary negative symptoms (eg, drug-induced parkinsonism or psychosis) rather than a direct effect on primary negative symptoms.[4]
According to a meta-analysis, olanzapine is superior to haloperidol in the likelihood of treatment success, improvement in the severity of mental disorders, and reduction in the severity of productive and negative disorders. Some studies have shown olanzapine to be superior to haloperidol in terms of its effect on cognitive function, but other studies have found no difference. Studies show that relapses occur significantly less when taking olanzapine than when taking haloperidol[4].
Pharmacology
Pharmacological action - antipsychotic, neuroleptic.
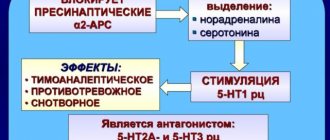
Has high affinity for serotonin 5-HT2A (dissociation constant Ki = 4 nM) and 5-HT2C (Ki = 11 nM), dopamine D1-4 (Ki = 11-31 nM), muscarinic M1-5 (Ki = 1.9 –25 nM), histamine H1 (Ki = 7 nM) and alpha1-adrenergic receptors (Ki = 19 nM). Weakly interacts with GABAA, benzodiazepine and beta-adrenergic receptors (Ki more than 10 µM). Under in vitro and in vivo conditions, it has a more pronounced affinity and activity for 5-HT2 receptors compared to D2 receptors. According to electrophysiological studies, it selectively reduces the excitability of mesolimbic dopaminergic neurons and has a slight effect on striatal nerve pathways involved in the regulation of motor functions. The antipsychotic effect is due to the blockade of serotonin 5-HT2 and dopamine receptors, anticholinergic effects are due to the blockade of M1−5 cholinergic receptors, drowsiness is due to the effect on histamine H1 receptors, orthostatic hypotension is due to the blockade of alpha1-adrenergic receptors.
Eliminates the productive symptoms of psychosis (delusions, hallucinations, thinking disorders, hostility, suspicion), smoothes out negative symptoms (emotional and social autism, introversion, poor speech). Dulls the severity of emotional experiences, weakens aggressiveness and impulsiveness of behavioral reactions, forms tolerance to the surrounding reality and reduces initiative. Relieves agitation and corrects behavioral and mental disorders in patients with mental disorders. Reduces the conditioned protective reflex (a test characterizing antipsychotic activity) in doses lower than doses that cause catalepsy. It is effective, especially in doses of 20–60 mg/day, in cases of schizophrenia refractory to treatment with typical neuroleptics: the effect gradually develops by the end of 2 months of treatment and then rapidly increases, reaching a maximum by the end of 4 months of therapy. There is evidence of effectiveness in depressive-delusional syndrome. Causes hyperprolactinemia (with long-term use), extrapyramidal disorders (rarely, mainly when using high doses), weight gain (450 g/week or more), which may persist after cessation of treatment.
It is well absorbed from the gastrointestinal tract; food intake does not affect the speed and completeness of absorption. Bioavailability is reduced by 40% due to the “first pass” effect through the liver. Cmax is reached after 5–8 hours. Equilibrium concentration is achieved after 1 non-daily dose and is twice the plasma concentration after a single dose. Plasma concentrations in the dose range of 1–20 mg change linearly and are proportional to the dose. Plasma protein binding - 93% (mainly with albumin and alpha1-acid glycoprotein). Passes through histohematic barriers, including the BBB. Distribution volume - about 1000 l. Biotransformed in the liver with the participation of isoenzymes CYP1A2 and CYP2D6 and flavin-containing monooxygenases to 10-N-glucuronide (44%) and 4-N-desmethylolanzapine (31%). Both metabolites are pharmacologically inactive at plasma concentrations within the therapeutic dose range of olanzapine. Slightly affects cytochrome P450 enzymes (the risk of undesirable pharmacokinetic interactions with other drugs is negligible). T1/2 depends on age and is 49–55 hours in patients over 65 years of age, 29–39 hours in patients under 65 years of age. Plasma clearance is 12–47 l/h (average 25 l/h) and decreases by 1.5 times in people over 65 years of age (compared to young people), by 30% in women (compared to men), by 40% in non-smokers (compared to smokers) and in cases of impaired liver function. Excreted by the kidneys (57%, unchanged - 7%) and intestines (30%). It is not excreted during dialysis (due to the large volume of distribution and high degree of binding to plasma proteins).
Carcinogenicity studies in mice and rats treated with olanzapine at doses 0.13–5 times the MRDC for 78 weeks to 2 years revealed an increase in the incidence of hemangioma and hemangiosarcoma of the liver (2–5 times the MRDC), adenoma and adenocarcinoma. breast (0.5–2 MRDH) associated with hyperprolactinemia. Reversible neutro- and lymphopenia, hemolytic anemia, and a decrease in the rate of increase in body weight were noted. No mutagenic properties were detected. Impaired fertility in male and female rats was detected at 11- and 1.5-fold excess of the MRDC, respectively. In female rats and rabbits treated during pregnancy with olanzapine at doses 9 and 30 times, respectively, higher than the MRDC, no teratogenic properties were detected. Early resorption of fetuses, a decrease in body weight and an increase in the number of non-viable fetuses were noted; The duration of pregnancy was extended when the MRPC was 5 times higher. Excreted in the breast milk of rats (there is no data on excretion in human milk).
Pharmacokinetics
Absorption
After oral administration, olanzapine is well absorbed from the gastrointestinal tract, Cmax in plasma is reached after 5-8. Olanzapine plasma concentrations have a linear dose response (ranging from 1 mg to 20 mg). Eating does not affect the absorption of olanzapine.
Distribution
At plasma concentrations from 7 to 1000 ng/ml, binding to plasma proteins, mainly albumin and a1-acid glycoprotein, is about 93%.
Metabolism
Olanzapine is metabolized in the liver by conjugation and oxidation. The main circulating metabolite is 10-N-glucuronide, which theoretically does not cross the BBB. Cytochrome P450 isoenzymes CYP1A2 and CYP2D6 are involved in the formation of N-desmethyl and 2-hydroxymethyl metabolites of olanzapine. Experimental studies on animals have shown that these metabolites have significantly less pronounced pharmacological activity in vivo
than olanzapine.
The main pharmacological activity of the drug is due to the parent substance - olanzapine. The activity of the cytochrome P450 isoenzyme CYP2D6 does not affect the level of metabolism of olanzapine. Elimination:
In healthy volunteers, after oral administration, the T1/2 half-life of olanzapine is 33 hours (21-54 hours for 5-95%), and the average plasma clearance is 26/hour (12-47 l/hour for 5-95%). About 57% of radiolabeled olanzapine is excreted in the urine, mainly in the form of metabolites.
Pharmacokinetics in special clinical situations
The pharmacokinetic parameters of olanzapine vary depending on gender, age, and the presence of smoking habits:
| Patient characteristics | T1/2 (h) | Plasma clearance (l/h) |
| Non-smokers | 38.6 | 18.6 |
| Smoking | 30.4 | 27.7 |
| Women | 36.7 | 18.9 |
| Men | 32.3 | 27.3 |
| Elderly (65 years and older) | 51.8 | 17.5 |
| Under 65 years old | 33.8 | 18.2 |
However, the degree of changes in T1/2 and clearance under the influence of each of these factors is significantly inferior to the degree of individual differences in these indicators.
There were no significant differences between the mean values of T1/2 and olanzapine clearance in patients with severely impaired renal function compared with individuals with normal renal function. In smoking patients with minor hepatic impairment, the clearance of olanzapine is lower than in non-smokers without such impairment. In a study involving subjects of European, Japanese and Chinese descent, there were no differences in the pharmacokinetics of olanzapine associated with race.
Olanzapine (Zyprexa) TB 10 mg No. 28 solution.
Olanzapine
Olanzapine
Release form of the drug Olanzapine
Pills.
Pharmacological action Olanzapine tablets
Antipsychotic drug (neuroleptic). Has affinity for serotonin (5-HT2A/2C, 5-HT3, 5-HT6), dopamine (D1, D2, D3, D4, D5), muscarinic (M1-5), adrenergic (α1) and histamine (H1) receptors .
In vitro, antagonism to 5-HT, dopamine and cholinergic receptors was detected. It has a more pronounced affinity and activity for serotonin 5-HT2 receptors compared to dopamine D2 receptors.
Selectively reduces the excitability of mesolimbic (A10) dopaminergic neurons, has a slight effect on striatal (A9) nerve pathways involved in the regulation of motor functions. Reduces the conditioned protective reflex in lower doses than doses that cause catalepsy.
Enhances the anti-anxiety effect during the “anxiolytic” test. Reliably reduces productive (including delusions, hallucinations) and negative symptoms.
PHARMACOKINETICS OF OLANZAPINE TABLETS
Suction and distribution
Absorption is high and does not depend on food intake; the time to reach Cmax after oral administration is 5-8 hours. When taken in a dose range of 1-20 mg, the plasma concentration changes linearly, proportional to the dose.
At plasma concentrations of 7-1000 ng/ml, protein binding is 93% (mainly with albumin and α1-acid glycoprotein).
Metabolism
Metabolized in the liver by conjugation and oxidation. The main circulating metabolite is 10-N-glucuronide, which does not cross the BBB.
Isoenzymes CYP1A2 and CYP2D6 are involved in the formation of N-desmethyl and 2-hydroxymethyl metabolites of olanzapine.
The main pharmacological activity of the drug is due to olanzapine, the activity of its metabolites is less pronounced.
Removal
Clearance - 26 l/h (12-47 l/h), T1/2 - 33 h (21-54 h). Excreted by the kidneys - 57% (mainly in the form of metabolites).
Pharmacokinetics in special clinical situations
Non-smokers (clearance - 18.6 l/h, T1/2 - 38.6 h), smokers (clearance - 27.7 l/h, T1/2 - 30.4 h), women (clearance - 18.9 l/h, T1/2 - 36.7 h) , men (clearance - 27.3 l/h, T1/2 - 32.3 h), patients 65 years and older (clearance - 17.5 l/h, T1/2 -51.8 h), patients under 65 years of age (clearance - 18.2 l /h, T1/2 - 33.8 h).
In smokers with minor liver dysfunction, clearance is lower than in non-smokers without liver dysfunction.
DOSAGE OF OLANZAPINE TABLETS
The drug is taken orally, regardless of food intake, at a dose of 5-20 mg/day.
For schizophrenia in adults , the recommended initial dose is 10 mg/day.
For acute mania associated with bipolar disorders in adults - 15 mg 1 time / day as monotherapy or 10 mg 1 time / day in combination with lithium or valproic acid (maintenance therapy at the same dose).
For depression associated with bipolar disorders in adults - 5 mg 1 time / day in combination with 20 mg fluoxetine (doses can be changed if necessary).
Elderly patients , patients with risk factors (including severe chronic renal failure or moderate liver failure), with a combination of risk factors (female gender, old age, non-smokers), in whom the metabolism of olanzapine may be slowed, it is recommended to reduce the initial dose to 5 mg/day.
OLANZAPINE TABLET OVERDOSE
Symptoms: tachycardia, agitation/aggression, articulation disorder, extrapyramidal disorders, disturbances of consciousness (from sedation to coma), delirium, convulsions, neuroleptic malignant syndrome, respiratory depression, aspiration, increased or decreased blood pressure, arrhythmias, cardiac and respiratory arrest.
Treatment: gastric lavage, administration of activated carbon, symptomatic treatment, maintaining respiratory function.
You should not use sympathomimetics (including epinephrine, dopamine), which are beta-adrenergic receptor agonists (stimulation of these receptors can aggravate the decrease in blood pressure).
The minimum dose for acute overdose with fatal outcome was 450 mg, the maximum dose with a favorable outcome (survival) was 1500 mg.
DRUG INTERACTIONS WITH OLANZAPINE TABLETS
Inducers or inhibitors of the CYP 1A2 isoenzyme may alter the metabolism of olanzapine.
The clearance of olanzapine increases in smoking patients and with simultaneous use of carbamazepine (CYP1A2 activity increases).
Ethanol did not affect the pharmacokinetics of olanzapine at steady state, however, taking ethanol together with olanzapine may be accompanied by an increase in the pharmacological effects of olanzapine (sedation).
Activated carbon reduces the bioavailability of olanzapine by up to 50-60%.
Fluoxetine (60 mg once or 60 mg daily for 8 days) increases the Cmax of olanzapine by 16% and reduces clearance by 16%, which has no clinical significance (no dose adjustment of olanzapine is required).
Fluvoxamine (CYP 1A2 inhibitor), reducing the clearance of olanzapine, increases the Cmax of olanzapine in non-smoking women by 54% and by 77% in smoking men, AUC by 52% and 108%, respectively (a dose reduction of olanzapine is required).
Olanzapine slightly inhibits the formation of valproic acid glucuronide (the main metabolic pathway). Valproic acid has little effect on the metabolism of olanzapine. A clinically significant pharmacokinetic interaction between olanzapine and valproic acid is unlikely.
PREGNANCY AND LACTATION
The drug should be prescribed with caution during pregnancy.
If it is necessary to prescribe the drug during lactation, breastfeeding should be discontinued.
SIDE EFFECTS OF OLANZAPINE TABLETS
The incidence of side effects is determined as follows: very often (≥ 10%), often (≥ 1% and <10%), infrequently (≥ 0.1% and <1%), rarely (≥ 0.01% and < 0.1%), very rare (<0.01%).
Somnolence and weight gain were very common in clinical studies; in 34% - hyperprolactinemia (mild and transient). Clinical manifestations of hyperprolactinemia were rare.
Common: dizziness, asthenia, akathisia, increased appetite, peripheral edema, orthostatic hypotension, dry oral mucosa, constipation.
Rarely: transient, asymptomatic increase in ALT, AST activity.
In isolated cases: an increase in plasma glucose levels of more than 200 mg/dl (suspicion of diabetes mellitus), 160 - 200 mg/dl (suspicion of hyperglycemia) in patients with an initial glucose concentration of less than 140 mg/dl.
Cases of increased levels of triglycerides (by 20 mg/dl from baseline), cholesterol (by 0.4 mg/dl from baseline), and asymptomatic eosinophilia (isolated cases) were observed.
In patients with psychosis due to dementia: very often - gait disturbance and falls; often - urinary incontinence and pneumonia.
In patients with psychosis induced by taking a dopamine agonist in Parkinson's disease: very often - increased symptoms of parkinsonism and hallucinations.
In patients with bipolar mania (receiving the drug in combination with lithium or valproic acid): very often - weight gain, dry oral mucosa, increased appetite, tremor; often - speech disorder.
The following are the side effects observed in clinical studies and post-marketing experience.
From the cardiovascular system: often - orthostatic hypotension; infrequently - bradycardia; very rarely - venous thromboembolism.
From the digestive system: often - constipation, dryness of the oral mucosa, increased appetite; rarely - hepatitis; very rarely - pancreatitis, jaundice.
Metabolism: often - peripheral edema; very rarely - diabetic coma, diabetic ketoacidosis. hyperglycemia, hypercholesterolemia, hypertriglyceridemia.
From the musculoskeletal system: very rarely - rhabdomyolysis.
From the nervous system: very often - drowsiness; often - akathisia, dizziness, asthenia; rarely - convulsions.
From the skin: rarely - rash.
From the genitourinary system: very rarely - priapism.
From the hematopoietic system: often - eosinophilia, rarely - leukopenia, very rarely - thrombocytopenia.
In terms of laboratory parameters: very often - hyperprolactinemia; often - increased activity of ALT, AST, hyperglycemia; very rarely - hyperbilirubinemia, increased alkaline phosphatase activity.
Other: very often - weight gain, infrequently - photosensitivity, very rarely - allergic reactions, withdrawal syndrome.
INDICATIONS OF OLANZAPINE TABLETS
- schizophrenia in adults (exacerbation, maintenance and long-term anti-relapse therapy), psychotic disorders with productive (including delusions, hallucinations, automatisms) and/or negative (emotional flatness, decreased social activity, impoverished speech) symptoms and concomitant affective disorders ;
- bipolar affective disorder in adults (monotherapy or in combination with lithium or valproic acid): acute manic or mixed episodes with/without psychotic manifestations and with/without rapid phase changes;
- relapse of bipolar disorder (if the drug is effective in treating the manic phase);
- depressive states associated with bipolar disorder (in combination with fluoxetine).
CONTRAINDICATIONS FOR OLANZAPINE TABLETS
- lactation period;
- children under 18 years of age;
- hypersensitivity to the components of the drug.
The drug should be prescribed with caution for liver failure, prostatic hyperplasia, angle-closure glaucoma, epilepsy, myelosuppression (including leukopenia, neutropenia), myeloproliferative diseases, hypereosinophilic syndrome, paralytic ileus, pregnancy.
SPECIAL INSTRUCTIONS FOR THE USE OF OLANZAPINE TABLETS
When treated with neuroleptics (including olanzapine), neuroleptic malignant syndrome may develop (hyperthermia, muscle rigidity, changes in mental status, autonomic disorders, including unstable pulse or blood pressure, tachycardia, cardiac arrhythmias, increased sweating; increased CPK activity , myoglobinuria as a result of rhabdomyolysis, acute renal failure).
If clinical manifestations of neuroleptic malignant syndrome are detected (including hyperthermia without other symptoms), discontinuation of olanzapine is required.
If signs of tardive dyskinesia develop, it is recommended to reduce the dose or discontinue olanzapine. Symptoms of tardive dyskinesia may increase or manifest after discontinuation of the drug.
When taking olanzapine (in studies), cerebrovascular events (stroke, transient ischemic attack), including deaths, were observed in elderly patients with psychosis due to dementia. These patients had previous risk factors (history of cerebrovascular disorders, transient ischemic attack, arterial hypertension, smoking), as well as concomitant diseases and/or medications that were temporally associated with cerebrovascular disorders. Olanzapine is not recommended for the treatment of patients with psychosis due to dementia.
Particular caution is required when ALT and/or AST levels are elevated in patients with liver failure or receiving treatment with potentially hepatotoxic drugs. Monitoring the patient and, if necessary, reducing the dose is required.
There is a higher prevalence of diabetes mellitus in patients with schizophrenia. Very rarely, cases of hyperglycemia, development of diabetes mellitus or exacerbation of pre-existing diabetes mellitus, ketoacidosis and diabetic coma have been reported. A causal relationship between antipsychotic drugs and these conditions has not been established. Clinical monitoring of patients with diabetes mellitus or with risk factors for its development is recommended.
Olanzapine should be used with caution in patients with a history of epileptic seizures or in the presence of factors that lower the seizure threshold.
Olanzapine should be used with caution in patients with a decrease in the number of leukocytes and/or neutrophils, with signs of suppression or toxic impairment of bone marrow function under the influence of drugs (history), with suppression of bone marrow function due to concomitant disease, radiotherapy or chemotherapy (in medical history); with hypereosinophilia or myeloproliferative disease.
The use of olanzapine in patients with clozapine-dependent neutropenia or agranulocytosis (history) was not accompanied by relapses of these disorders.
It is recommended to exercise caution when prescribing olanzapine to patients with symptomatic prostatic hypertrophy, paralytic ileus, and angle-closure glaucoma.
Olanzapine exhibits dopamine antagonism and, theoretically, may inhibit the effects of levodopa and dopamine agonists.
Caution should be exercised when using olanzapine in combination with other centrally acting drugs and ethanol.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
USE OF OLANZAPINE TABLETS IN ELDERLY AGES
Elderly patients , patients with risk factors (including severe chronic renal failure or moderate liver failure), with a combination of risk factors (female gender, old age, non-smokers), in whom the metabolism of olanzapine may be slowed, it is recommended to reduce the initial dose to 5 mg/day.
USE OF OLANZAPINE TABLETS IN CHILDREN
Contraindicated in children and adolescents under 18 years of age.
Storage conditions for Olanzapine tablets
The drug should be stored in a dry place, protected from light, out of reach of children at a temperature not exceeding 25°C. Shelf life: 2 years.
Indications
- treatment of exacerbations; maintenance and long-term anti-relapse therapy for schizophrenia and other psychotic disorders with pronounced productive (including delusions, hallucinations, automatism) and/or negative (including emotional flatness, decreased social activity, impoverished speech) symptoms, as well as concomitant affective disorders;
- treatment of acute manic or mixed attacks in bipolar affective disorder.
- can be used to treat stimulant psychoses[2].
Olanzapine-Canon tablets 10 mg No. 28
special instructions
Clinical improvement may take several days and requires patient monitoring.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome (NMS) (a potentially lethal symptom complex) can develop during treatment with any antipsychotics, including the drug Olanzapine Canon, but to date there is no data confirming a reliable connection between taking olanzapine and the development of this condition. Clinical manifestations of neuroleptic malignant syndrome include significant increase in body temperature, muscle rigidity, changes in mental status and autonomic disturbances (unstable pulse or blood pressure, tachycardia, cardiac arrhythmias, increased sweating). Additional features may include increased creatine phosphokinase levels, myoglobinuria (rhabdomyolysis), and acute renal failure. Clinical manifestations of neuroleptic malignant syndrome or a significant increase in body temperature without other symptoms of neuroleptic malignant syndrome require discontinuation of all antipsychotics, including the drug Olanzapine Canon.
Tardive dyskinesia
Treatment with olanzapine is less often accompanied by the development of dyskinesia, requiring drug correction, than with the use of haloperidol. However, the risk of tardive dyskinesia should be taken into account during long-term therapy with antipsychotics. If signs of tardive dyskinesia develop, it is recommended to reduce the dose or discontinue Olanzapine Canon. It should be taken into account that when switching to the drug Olanzapine Canon, symptoms of tardive dyskinesia may develop as a result of immediate withdrawal of previous therapy. Symptoms of tardive dyskinesia may increase or manifest after discontinuation of the drug.
Parkinson's disease
Efficacy when used in Parkinson's disease does not exceed placebo (for the purpose of relieving iatrogenic psychoses). The use of the drug Olanzapine Canon is not recommended in the treatment of psychoses induced by taking dopamine receptor agonists in Parkinson's disease; such patients experience increased symptoms of parkinsonism and hallucinations.
Experience of use in elderly patients with psychosis due to dementia
Cerebrovascular adverse events (eg, stroke, transient ischemic attack), including deaths, have been reported in elderly patients with psychosis associated with dementia. In placebo-controlled studies, there was a higher incidence of cerebrovascular adverse events in patients in the olanzapine group compared with the placebo group. These patients had previous risk factors (history of cerebrovascular disorders, transient ischemic attack, arterial hypertension, smoking), as well as concomitant diseases and/or medications that were temporally associated with cerebrovascular disorders.
The effectiveness of olanzapine in elderly patients with psychosis due to dementia has not been established. The main risk factors for increased mortality in this group of patients when treated with olanzapine are age 80 years, sedation, concomitant use with benzodiazepines, or the presence of pulmonary pathology (eg, pneumonia with or without aspiration). There are insufficient data to establish differences in the incidence of cerebrovascular events and/or mortality (compared with placebo) and risk factors between oral and intramuscular olanzapine in this group of patients. The drug Olanzapine Canon is not recommended for the treatment of patients with psychosis due to dementia.
Development of the risk of sudden death
Clinical experience with all antipsychotics, including olanzapine, has shown a similar dose-dependent two-fold increase in the risk of death due to acute heart failure compared with death due to acute heart failure in patients not taking antipsychotics.
Duration of the QT interval
Infrequently, a clinically significant increase in the QT interval was observed in patients receiving olanzapine, while there were no significant differences with placebo in the incidence of adverse cardiac events. However, as with the use of other antipsychotic drugs, it is recommended to exercise caution when prescribing Olanzapine Canon in combination with drugs that can prolong the QT interval, especially in elderly patients, with congenital prolongation of the QT interval, with congestive heart failure, myocardial hypertrophy, hypokalemia and hypomagnesemia.
Postural hypotension
Postural hypotension is uncommon in elderly patients. Just as when using other antipsychotics, when Olanzapine Canon is prescribed to patients over 65 years of age, it is recommended to monitor blood pressure.
Thromboembolism
The development of venous thromboembolism during olanzapine therapy is extremely rare. The presence of a cause-and-effect relationship between taking olanzapine and venous thromboembolism has not been established. However, given that patients with schizophrenia often have acquired risk factors for venous thromboembolism, it is necessary to conduct a cumulative assessment of all possible risk factors for the development of this complication, including immobilization of patients, and take the necessary preventive measures.
Liver dysfunction
In some cases, taking olanzapine, usually in the early stages of therapy, is accompanied by a transient, asymptomatic increase in hepatic transaminases (AST and ALT) in the blood serum. Rare cases of hepatitis have been reported. In very rare cases, hepatic cholestasis and other mixed liver damage have been observed. Particular caution is required when serum AST and/or ALT levels increase in patients with impaired liver function, with limited liver functional reserve, or in patients receiving treatment with potentially hepatotoxic drugs. If AST and/or ALT levels increase during treatment with olanzapine, careful monitoring of the patient is required and, if necessary, a reduction in the dose of Olanzapine Canon. If hepatitis, including hepatocellular, cholestatic or mixed, is detected, Olanzapine Canon should be discontinued.
Hyperglycemia and diabetes mellitus
There is a higher prevalence of diabetes mellitus in patients with schizophrenia. As with some other antipsychotic drugs, very rare cases of hyperglycemia, diabetes mellitus, exacerbation of pre-existing diabetes mellitus, ketoacidosis and diabetic coma have been reported. A cause-and-effect relationship between antipsychotic drugs and these conditions has not been established. Close clinical monitoring of patients with diabetes mellitus and patients with risk factors for developing diabetes mellitus is recommended.
For all groups of patients, regardless of body mass index, a clinically significant increase in body weight was observed. An increase of 7% or more from the mean after a short course of treatment (average duration of 47 days) was very common (22.2%), an increase of 15% or more was common (4.2%), and an increase of 25% or more was infrequent (0.8%).
In patients receiving longer treatment (at least 48 weeks), increases of 7%, 15%, and 25% were very common (64.4%, 31.7%, 12.3%, respectively).
Changes in lipid profile
In patients treated with olanzapine, undesirable changes in the lipid spectrum are observed. Monitoring of the lipid profile and clinical observation are recommended.
Epileptic seizures
Olanzapine Canon should be used with caution in patients with a history of epileptic seizures or those exposed to factors that lower the seizure threshold. In these patients, seizures are rare when treated with olanzapine.
Hematological changes
As with other antipsychotics, caution should be exercised when treating olanzapine in patients with a decreased number of leukocytes and/or neutrophils in the peripheral blood due to various reasons, with signs of depression or toxic impairment of bone marrow function under the influence of drugs in the anamnesis, with depression of function bone marrow due to concomitant disease, history of radiotherapy or chemotherapy, hypereosinophilia or myeloproliferative disease.
In clinical studies, the use of olanzapine in patients with a history of clozapine-dependent neutropenia or agranulocytosis was not accompanied by relapses of these disorders. The development of neutropenia has been reported primarily during concomitant therapy with olanzapine and valproic acid.
Anticholinergic activity
Olanzapine therapy is rarely associated with anticholinergic side effects. However, clinical experience with olanzapine in patients with concomitant diseases is limited, so caution is recommended when prescribing Olanzapine Canon to patients with clinically significant prostatic hypertrophy, paralytic ileus and similar conditions.
Dopaminergic antagonism
In vitro
Olanzapine exhibits dopamine receptor antagonism and, like other antipsychotics, may theoretically inhibit the effects of levodopa and dopamine agonists.
General activity in relation to the central nervous system
Given the primary CNS effects of olanzapine, caution should be exercised when using olanzapine in combination with other centrally acting drugs and alcohol.
Suicide
The risk of suicide attempts in patients with schizophrenia and bipolar disorder type 1 is determined by these diseases themselves. In this regard, during pharmacotherapy, careful monitoring of those patients whose risk of suicide is especially high is required. When prescribing Olanzapine Canon, one should strive to minimize the number of tablets taken by the patient in order to reduce the risk of overdose.
Cancellation of therapy
In case of abrupt withdrawal of olanzapine, sweating, insomnia, tremor, nausea and vomiting rarely (0.01-0.1%) develop. When discontinuing the drug, a gradual dose reduction is recommended.
Children and teenagers under 18 years of age
Olanzapine is not recommended for use in children and adolescents under 18 years of age due to the lack of sufficient data on efficacy and safety. In short-term studies that were conducted in adolescents 13-17 years of age, a greater increase in body weight and changes in lipid and prolactin concentrations were noted. than in similar studies in adults.
Dosage regimen
Russian-made olanzapine
For schizophrenia and similar psychotic disorders, the recommended initial dose of the drug is 10 mg/day, once. Olanzapine can be taken with or without food. Therapeutic doses range from 5-20 mg/day. The daily dose must be selected individually depending on the clinical condition of the patient. Increasing the dose above the standard 10 mg/day is recommended only after an appropriate clinical examination of the patient.
For acute mania, the recommended initial dose of the drug is 15 mg/day, once. Therapeutic doses of olanzapine range from 5 to 20 mg/day.
In elderly patients, as well as in cases of severe renal failure or moderate liver failure, the drug is prescribed at an initial dose of 5 mg/day.
A reduction in the initial dose is recommended for patients with a combination of factors (female patients, old age, non-smokers) that may slow down the metabolism of olanzapine.
Description of withdrawal syndrome
Withdrawal syndrome, or withdrawal reaction, is a set of clinical manifestations associated with stopping taking a drug. The severity of the patient’s condition depends on the treatment regimen (dose, regimen, duration), but the main role is played by the individual characteristics of the person. All other things being equal, the severity of symptoms will vary from person to person.
Causes of withdrawal
The pharmacokinetics of olanzapine largely depends on age, activity of the underlying disease, condition of internal organs and gender. The following risk factors for the development of neuroleptic withdrawal syndrome are identified:
- abruptly stopping the drug
- long course of therapy with one drug
- dose reduction not agreed with the doctor
- violation of the treatment regimen
- short duration of pharmacotherapy, especially when using maximum daily doses - less than 6 months, sometimes 2 weeks are enough
- simultaneous discontinuation of other medications that correct mental status
- replacing one antipsychotic with another due to stabilization of the patient’s condition, or vice versa, the appearance of new symptoms
Side effects
Very common (>=10%): drowsiness, weight gain. In 34% of patients, an increase in the concentration of prolactin in the blood plasma was observed, which was mild and transient (the average value of the maximum concentrations of prolactin did not reach the upper limit of normal and was not statistically significantly different from placebo). Clinical manifestations of hyperprolactinemia associated with olanzapine (i.e., gynecomastia, galactorrhea, breast enlargement) have been reported rarely. In most patients, normalization of prolactin levels was observed without discontinuation of olanzapine. Another very common (>=10%) side effect associated with the use of olanzapine in clinical trials in patients with dementia of the Alzheimer's type was gait disturbances.
Often (<10% and >=1%): dizziness, asthenia, akathisia, increased appetite, peripheral edema, orthostatic hypotension, dry mouth, constipation.
Rarely, a transient, asymptomatic increase in liver transaminases (ALT, AST) was observed; in isolated cases - an increase in plasma glucose levels to >=200 mg/dL (suspicion of diabetes), as well as >=160 mg/dL, but <200 mg/dL (suspicion of hyperglycemia) in patients with an initial glucose level of < =140 mg/dl; Some patients had asymptomatic eosinophilia.
The table below summarizes the main side effects and their frequency reported during clinical trials and/or post-marketing experience.
| System/Side Effect | Frequency | ||||
| ≥ 10 % | < 10% and ≥ 1% | <1% and ≥0.1% | <0.1% and ≥ 0.01% | <0.01 % | |
| Body as a whole | |||||
| 2Asthenia | • | ||||
| 2Increased sensitivity to light | • | ||||
| 1Increase in body weight | • | ||||
| The cardiovascular system | |||||
| 2Bradycardia | • | ||||
| 1Orthostatic hypotension | • | ||||
| Digestive system | |||||
| 2Constipation | • | ||||
| 2Dry mouth | • | ||||
| 3Hepatitis | • | ||||
| 2Increased appetite | • | ||||
| Metabolic disorders | |||||
| 3Diabetic coma | • | ||||
| 3.4 Diabetic ketoacidosis | • | ||||
| 3Hyperglycemia | • | ||||
| 1Peripheral edema | • | ||||
| Nervous system | |||||
| 4 Gait disturbance | • | ||||
| 2Akathisia | • | ||||
| 2Dizziness | • | ||||
| 3 Seizures | • | ||||
| 2Drowsiness | • | ||||
| 3Rash | • | ||||
| Genitourinary system | |||||
| 3Priapism | • | ||||
| Clinical biochemistry | |||||
| 1Increase in ALT | • | ||||
| 1Increase in AST | • | ||||
| 1Increase in prolactin | • | ||||
| 1 Isolated cases of increased glucose levels >=160 mg/dL, but <200 mg/dL (suspected hyperglycemia) | • | ||||
| 1 Isolated cases of increased glucose levels >=200 mg/dl (suspicious diabetes mellitus) | • | ||||
| Hematology | |||||
| 1Eosinophilia | • | ||||
| 3Leukopenia | • | ||||
| 3Thrombocytopenia | • | ||||
1 - Evaluation of indicators from the clinical trial database. 2 - Adverse events recorded in the clinical trial database. 3 - Side effects recorded spontaneously during post-marketing studies. 4 - Side effects identified in clinical studies in patients with dementia of the Alzheimer's type. 5 - In the COSTART classification it is designated as diabetic acidosis.
Dysarthria, swelling of the nasal mucosa, tremor, stiff neck muscles, and insomnia were also observed during olanzapine therapy [5].
Olanzapine has a comparatively lower risk of somnolence, orthostatic hypotension and tachycardia than some other atypical antipsychotics (primarily clozapine and quetiapine) and a lower risk of prolactin elevation than risperidone[4]. However, in a small percentage of patients (10-15%), the effects of sedation and drowsiness persist and last for months, which impedes the quality of social recovery[6].
Obesity and sedation with olanzapine are most pronounced in children and adolescents[7].
Olanzapine may cause symptoms of obsessive-compulsive disorder due to its strong antiserotonergic effect[8]. If patients have severe depressive and obsessive-compulsive symptoms, olanzapine can enhance these phenomena[9].
When taking olanzapine, the development of anticholinergic syndrome is possible [10].
Metabolic and endocrine disorders
Olanzapine has the highest risk of obesity and metabolic disorders compared to other antipsychotics[11]. In patients taking 15 mg of olanzapine per day, after a year of therapy, body weight increases by an average of 11.8 kg. An increase in weight during treatment with this drug is noted up to 12% of the initial weight of patients [12]. Sometimes the weight gain is 20-45 kg[13].
Consequences of antipsychotic-induced obesity include an increased risk of coronary heart disease, hypertension, cancer, diabetes mellitus, osteoarthritis, sleep apnea[4], cholelithiasis, myocardial infarction and stroke[14]. The use of olanzapine statistically significantly increases the risk of developing diabetes mellitus by 6 times[11].
Diabetic ketoacidosis, a relatively rare and extremely dangerous complication of diabetes, can also be caused by taking olanzapine. There have been numerous cases where diabetic ketoacidosis developed suddenly, in the absence of previously diagnosed diabetes. The possibility of diabetic ketoacidosis must always be kept in mind: its mental manifestations can easily be confused with symptoms of schizophrenia[4].
Animal studies
In a placebo-controlled study, groups of six macaques received olanzapine and haloperidol at therapeutic dosages for about two years. In post-mortem analysis, macaques treated with neuroleptics showed a similar decrease in both brain volume and weight, reaching 8-11%[15]. Upon further study of preserved samples, it was shown that the decrease in gray matter volume was primarily due to a decrease in the number of astrocytes, and secondly, oligodendrocytes,[16] while the density of neurons increased, although their number remained unchanged.
Zyprexa®
Clinical improvement with antipsychotic treatment may take several days to several weeks and requires careful monitoring of the patient.
Suicide
The risk of suicide attempts in patients with schizophrenia and bipolar disorder type 1 is determined by these diseases themselves. In this regard, during pharmacotherapy, careful monitoring of those patients whose risk of suicide is especially high is required. When prescribing olanzapine, efforts should be made to minimize the number of tablets taken by the patient in order to reduce the risk of overdose.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome (NMS) can develop during treatment with any antipsychotic drug, including olanzapine. Clinical manifestations of neuroleptic malignant syndrome include significant increase in body temperature, muscle rigidity, changes in mental status and autonomic disturbances (unstable pulse or blood pressure, tachycardia, arrhythmias, increased sweating). Additional features may include increased creatine phosphokinase activity, myoglobinuria (rhabdomyolysis), and acute renal failure. Clinical manifestations of neuroleptic malignant syndrome or a significant unexplained increase in body temperature without other symptoms of neuroleptic malignant syndrome require discontinuation of all antipsychotics, including olanzapine.
Tardive dyskinesia
In comparative studies lasting more than 6 weeks, treatment with olanzapine was significantly less likely to be accompanied by the development of dyskinesia requiring drug correction than the use of haloperidol. However, the increased risk of tardive dyskinesia should be taken into account with long-term therapy with antipsychotics. If signs of tardive dyskinesia develop, it is recommended to reduce the dose or discontinue olanzapine. Symptoms of tardive dyskinesia may increase or manifest after discontinuation of the drug.
Psychosis associated with dementia and/or conduct disorders
Olanzapine is not indicated for the treatment of psychosis associated with dementia and/or behavioral disorders and is not recommended for use in this group of patients due to the high mortality rate and risk of cerebrovascular accidents. In placebo-controlled studies (duration 6-12 weeks) in elderly patients (mean age 78 years) with psychosis associated with dementia and/or conduct problems, there was a two-fold higher incidence of death in patients in the olanzapine group compared with those in the olanzapine group. placebo group (3.5% and 1.5%, respectively). The higher mortality rate was not associated with the dose of olanzapine (mean dose 4.4 mg) or duration of treatment. Risk factors that may predispose this group of patients to higher mortality when treated with olanzapine include age ≥ 65 years, dysphagia, sedation, malnutrition (wasting), dehydration, concomitant use with benzodiazepines, or the presence of pulmonary pathology (eg, pneumonia with with or without aspiration).
Cerebrovascular adverse events (eg, stroke, transient ischemic attack), including deaths, were observed in the same studies of olanzapine in elderly patients. In placebo-controlled studies, there was a threefold higher incidence of cerebrovascular adverse events in patients in the olanzapine group compared with the placebo group (1.3% versus 0.4%, respectively). All patients with cerebrovascular disorders had previous risk factors for the development of cerebrovascular adverse events: age ≥75 years, vascular or mixed dementia, a previous case of cerebrovascular adverse event or transient ischemic attack, arterial hypertension, smoking, as well as concomitant diseases and/or medications, temporally associated with cerebrovascular adverse events. The effectiveness of olanzapine was not established in these studies.
Parkinson's disease
The use of olanzapine is not recommended in the treatment of psychosis induced by dopamine receptor agonists in Parkinson's disease.
In clinical trials in patients with drug-induced psychosis (dopamine receptor agonist) for Parkinson's disease, increased parkinsonian symptoms and hallucinations were very common (≥10%) and at a higher frequency than in the placebo group. Olanzapine was not superior to placebo in treating psychotic symptoms in patients with Parkinson's disease. In these clinical studies, patients were required to take antiparkinsonian drugs (dopamine agonists) at the lowest effective dose and continue to take them at the same dose throughout the study. The starting dose of olanzapine was 2.5 mg per day and could be increased to 15 mg per day as recommended by the physician.
Liver dysfunction
In some cases, taking olanzapine, usually in the early stages of therapy, was accompanied by a transient, asymptomatic increase in hepatic aminotransferases (aspartate aminotransferase and alanine aminotransferase) in the blood serum. Rare cases of hepatitis have been reported. In very rare cases, hepatic cholestasis and other associated liver damage have been observed. Particular caution is required when serum aspartate aminotransferase and/or alanine aminotransferase activity increases in patients with impaired liver function, with limited liver functional reserve, or in patients receiving treatment with potentially hepatotoxic drugs.
Hyperglycemia and diabetes mellitus
Patients with schizophrenia have a higher prevalence of diabetes. As with some other antipsychotic drugs, cases of hyperglycemia, diabetes, exacerbation of pre-existing diabetes, ketoacidosis and diabetic coma have been reported. Close clinical monitoring of patients with diabetes and patients with risk factors for developing diabetes is recommended according to the following guidelines: measurement of blood glucose concentrations at baseline, 12 weeks after starting olanzapine, and annually thereafter. Patients taking antipsychotic drugs, including Zyprexa®, should be monitored for signs and symptoms of hyperglycemia (such as polydipsia, polyuria, polyphagia, weakness). Patients with diabetes mellitus or risk factors for diabetes mellitus require regular monitoring of blood glucose concentrations. Regular monitoring of body weight is necessary: before starting treatment, 4, 8 and 12 weeks after starting olanzapine, and subsequently every 3 months.
Change in lipid profile
During placebo-controlled studies, undesirable lipid changes were observed in patients receiving olanzapine (see Adverse Reactions), especially in patients with dyslipidemia and in patients with risk factors for developing lipid disorders. Patients taking antipsychotic medications, including Zyprexa, should have their lipid profiles monitored regularly as recommended: before starting treatment, 12 weeks after starting olanzapine, and every 5 years thereafter.
Sudden cardiac death
Sudden deaths have been reported in post-marketing surveillance of olanzapine. A retrospective observational study found an approximately twofold increase in the risk of sudden death in the olanzapine group compared with the group of patients not receiving antipsychotics. In this study, these results for olanzapine were comparable to those for the other atypical antipsychotics included in the analysis. Spontaneous reports of sudden death during post-marketing surveillance have been rare.
Convulsions
Olanzapine should be used with caution in patients with a history of seizures or exposure to factors that lower the seizure threshold. Cases of seizures were uncommon in patients taking olanzapine, and in most of these cases, the patients had a history of seizures or risk factors for seizures.
Hematological changes
As with the use of other antipsychotics, caution should be exercised when treating patients with olanzapine with a low number of leukocytes and/or neutrophils in the peripheral blood due to various reasons; with signs of suppression or toxic impairment of bone marrow function under the influence of drugs in the anamnesis; with suppression of bone marrow function due to concomitant disease, radiotherapy or chemotherapy in history; with hypereosinophilia or myeloproliferative disease.
In clinical studies, the use of olanzapine in patients with a history of clozapine-dependent neutropenia or agranulocytosis was not accompanied by relapses of these disorders.
Cancellation of therapy
In case of immediate withdrawal of olanzapine, sudden development of sweating, insomnia, tremor, anxiety, nausea and vomiting was rarely (≥ 0.01% and < 0.1%) reported.
QT interval duration
In clinical studies, clinically significant prolongation of the QT interval (QT interval adjusted by Fridericius [QTcF] > 500 ms in patients with baseline QTcF < 500 ms) was observed infrequently (0.1% to 1%) in patients receiving olanzapine, while no significant differences with placebo in the incidence of adverse cardiac events. However, as with other antipsychotics, caution is recommended when prescribing olanzapine in combination with drugs that can prolong the QT interval, especially in elderly patients with congenital prolongation of the QT interval, congestive heart failure, myocardial hypertrophy, hypokalemia and hypomagnesemia .
General activity in relation to the central nervous system (CNS)
Given the primary action of olanzapine on the central nervous system, caution should be exercised when using olanzapine in combination with other centrally acting drugs and alcohol.
Postural hypotension
Postural hypotension was observed infrequently in clinical studies of olanzapine in the elderly. As with other antipsychotics, periodic monitoring of blood pressure is recommended when olanzapine is prescribed to patients over 65 years of age.
Thromboembolism
Infrequently (≥ 0.1% and < 1%) cases of a temporal association between the development of venous thromboembolism and olanzapine therapy have been reported. The presence of a cause-and-effect relationship between olanzapine and venous thromboembolism has not been established. However, given that patients with schizophrenia often have acquired risk factors for venous thromboembolism, it is necessary to conduct a cumulative assessment of all possible risk factors for the development of this complication, including immobilization of patients, and take the necessary preventive measures.
Lactose
Zyprexa® tablets contain lactose. Patients with rare hereditary galactose intolerance, lactase intolerance and glucose-galactose malabsorption should not take olanzapine.
Anticholinergic activity
Although olanzapine exhibited anticholinergic activity in in vitro studies, olanzapine therapy was rarely associated with anticholinergic side effects in clinical studies. However, clinical experience with olanzapine in patients with comorbidities is limited, and caution is recommended when prescribing olanzapine to patients with clinically significant prostatic hypertrophy, paralytic ileus, angle-closure glaucoma, and similar conditions.
Dopaminergic antagonism
In vitro
Olanzapine exhibits dopamine antagonism and, like other antipsychotics, may theoretically inhibit the effects of levodopa and dopamine agonists.
Contraindications
Liver dysfunction, epilepsy, myelosuppression, prostatic hypertrophy, paralytic intestinal obstruction, childhood and adolescence (up to 18 years) [17], hypersensitivity to the drug, severe depression of the central nervous system, coma. With caution - Parkinson's disease, diabetes mellitus, concomitant use of drugs that prolong the QT interval[17], renal failure, angle-closure glaucoma, seizures (history), myeloproliferative diseases, hypereosinophilic syndrome, pregnancy, lactation.
Should not be prescribed to drivers and people engaged in heavy mental and physical labor.
Olanzapine Canon
Clinical improvement may take several days and requires patient monitoring.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome (NMS) (a potentially lethal symptom complex) can develop during treatment with any antipsychotics, including the drug Olanzapine Canon, but to date there is no data confirming a reliable connection between taking olanzapine and the development of this condition. Clinical manifestations of neuroleptic malignant syndrome include significant increase in body temperature, muscle rigidity, changes in mental status and autonomic disturbances (unstable pulse or blood pressure, tachycardia, cardiac arrhythmias, increased sweating). Additional features may include increased creatine phosphokinase levels, myoglobinuria (rhabdomyolysis), and acute renal failure. Clinical manifestations of neuroleptic malignant syndrome or a significant increase in body temperature without other symptoms of neuroleptic malignant syndrome require discontinuation of all antipsychotics, including the drug Olanzapine Canon.
Tardive dyskinesia
Treatment with olanzapine is less often accompanied by the development of dyskinesia, requiring drug correction, than with the use of haloperidol. However, the risk of tardive dyskinesia should be taken into account during long-term therapy with antipsychotics. If signs of tardive dyskinesia develop, it is recommended to reduce the dose or discontinue Olanzapine Canon. It should be taken into account that when switching to the drug Olanzapine Canon, symptoms of tardive dyskinesia may develop as a result of immediate withdrawal of previous therapy. Symptoms of tardive dyskinesia may increase or manifest after discontinuation of the drug.
Parkinson's disease
Efficacy when used in Parkinson's disease does not exceed placebo (for the purpose of relieving iatrogenic psychoses). The use of the drug Olanzapine Canon is not recommended in the treatment of psychoses induced by taking dopamine receptor agonists in Parkinson's disease; such patients experience increased symptoms of parkinsonism and hallucinations.
Experience of use in elderly patients with psychosis due to dementia
Cerebrovascular adverse events (eg, stroke, transient ischemic attack), including deaths, have been reported in elderly patients with psychosis associated with dementia. In placebo-controlled studies, there was a higher incidence of cerebrovascular adverse events in patients in the olanzapine group compared with the placebo group. These patients had previous risk factors (history of cerebrovascular disorders, transient ischemic attack, arterial hypertension, smoking), as well as concomitant diseases and/or medications that were temporally associated with cerebrovascular disorders.
The effectiveness of olanzapine in elderly patients with psychosis due to dementia has not been established. The main risk factors for increased mortality in this group of patients when treated with olanzapine are age ≥ 80 years, sedation, concomitant use with benzodiazepines, or the presence of pulmonary pathology (eg, pneumonia with or without aspiration). There are insufficient data to establish differences in the incidence of cerebrovascular events and/or mortality (compared with placebo) and risk factors between oral and intramuscular olanzapine in this group of patients. The drug Olanzapine Canon is not recommended for the treatment of patients with psychosis due to dementia.
Development of the risk of sudden death
Clinical experience with all antipsychotics, including olanzapine, has shown a similar dose-dependent two-fold increase in the risk of death due to acute heart failure compared with death due to acute heart failure in patients not taking antipsychotics.
Duration of the QT interval
Infrequently, a clinically significant increase in the QT interval was observed in patients receiving olanzapine, while there were no significant differences with placebo in the incidence of adverse cardiac events. However, as with the use of other antipsychotic drugs, it is recommended to exercise caution when prescribing Olanzapine Canon in combination with drugs that can prolong the QT interval, especially in elderly patients, with congenital prolongation of the QT interval, with congestive heart failure, myocardial hypertrophy, hypokalemia and hypomagnesemia.
Postural hypotension
Postural hypotension is uncommon in elderly patients. Just as when using other antipsychotics, when Olanzapine Canon is prescribed to patients over 65 years of age, it is recommended to monitor blood pressure.
Thromboembolism
The development of venous thromboembolism during olanzapine therapy is extremely rare. The presence of a cause-and-effect relationship between taking olanzapine and venous thromboembolism has not been established. However, given that patients with schizophrenia often have acquired risk factors for venous thromboembolism, it is necessary to conduct a cumulative assessment of all possible risk factors for the development of this complication, including immobilization of patients, and take the necessary preventive measures.
Liver dysfunction
In some cases, taking olanzapine, as a rule, in the early stages of therapy is accompanied by a transient, asymptomatic increase in liver transaminases (AST and ALT) in the blood serum. Rare cases of hepatitis have been reported. In very rare cases, hepatic cholestasis and other mixed liver damage have been observed. Particular caution is required when serum AST and/or ALT levels increase in patients with impaired liver function, with limited liver functional reserve, or in patients receiving treatment with potentially hepatotoxic drugs. If AST and/or ALT levels increase during treatment with olanzapine, careful monitoring of the patient is required and, if necessary, a reduction in the dose of Olanzapine Canon. If hepatitis, including hepatocellular, cholestatic or mixed, is detected, Olanzapine Canon should be discontinued.
Hyperglycemia and diabetes mellitus
There is a higher prevalence of diabetes mellitus in patients with schizophrenia. As with some other antipsychotic drugs, very rare cases of hyperglycemia, diabetes mellitus, exacerbation of pre-existing diabetes mellitus, ketoacidosis and diabetic coma have been reported. A cause-and-effect relationship between antipsychotic drugs and these conditions has not been established. Close clinical monitoring of patients with diabetes mellitus and patients with risk factors for developing diabetes mellitus is recommended.
For all groups of patients, regardless of body mass index, a clinically significant increase in body weight was observed. An increase of 7% or more from the mean after a short course of treatment (average duration of 47 days) was very common (22.2%), an increase of 15% or more was common (4.2%), and an increase of 25% or more was infrequent (0.8%).
In patients receiving longer treatment (at least 48 weeks), increases of ≥7%, ≥15%, and ≥25% were very common (64.4%, 31.7%, 12.3%, respectively).
Changes in lipid profile
In patients treated with olanzapine, undesirable changes in the lipid spectrum are observed. Monitoring of the lipid profile and clinical observation are recommended.
Epileptic seizures
Olanzapine Canon should be used with caution in patients with a history of epileptic seizures or those exposed to factors that lower the seizure threshold. In these patients, seizures are rare when treated with olanzapine.
Hematological changes
As with the use of other antipsychotics, caution should be exercised when treating patients with olanzapine with a low number of leukocytes and/or neutrophils in the peripheral blood due to various reasons; with signs of suppression or toxic impairment of bone marrow function under the influence of drugs in the anamnesis; with suppression of bone marrow function due to concomitant disease, radiotherapy or chemotherapy in history; with hypereosinophilia or myeloproliferative disease.
In clinical studies, the use of olanzapine in patients with a history of clozapine-dependent neutropenia or agranulocytosis was not accompanied by relapses of these disorders. The development of neutropenia has been reported primarily during concomitant therapy with olanzapine and valproic acid.
Anticholinergic activity
Olanzapine therapy is rarely associated with anticholinergic side effects. However, clinical experience with olanzapine in patients with concomitant diseases is limited, so caution is recommended when prescribing Olanzapine Canon to patients with clinically significant prostatic hypertrophy, paralytic ileus and similar conditions.
Dopaminergic antagonism
In vitro
Olanzapine exhibits dopamine receptor antagonism and, like other antipsychotics, may theoretically inhibit the effects of levodopa and dopamine agonists.
General activity in relation to the central nervous system
Given the primary CNS effects of olanzapine, caution should be exercised when using olanzapine in combination with other centrally acting drugs and alcohol.
Suicide
The risk of suicide attempts in patients with schizophrenia and bipolar disorder type 1 is determined by these diseases themselves. In this regard, during pharmacotherapy, careful monitoring of those patients whose risk of suicide is especially high is required. When prescribing Olanzapine Canon, one should strive to minimize the number of tablets taken by the patient in order to reduce the risk of overdose.
Cancellation of therapy
In case of abrupt withdrawal of olanzapine, sweating, insomnia, tremor, nausea and vomiting rarely (0.01-0.1%) develop. When discontinuing the drug, a gradual dose reduction is recommended.
Children and teenagers under 18 years of age
Olanzapine is not recommended for use in children and adolescents under 18 years of age due to the lack of sufficient data on efficacy and safety. In short-term studies that were conducted in adolescents 13-17 years of age, a greater increase in body weight and changes in lipid and prolactin concentrations were noted. than in similar studies in adults.
special instructions
At the beginning of treatment, especially when selecting the dosage, observation is necessary: extrapyramidal side effects, orthostatic hypotension and reflex tachycardia, drowsiness, weight gain, hyperglycemia and hyperlipoproteinemia are possible. The risk of orthostatic hypotension increases when olanzapine is combined with benzodiazepines. Drowsiness often develops at the beginning of treatment, so it is better to take the drug at night[4].
Because of the potential for agranulocytosis, it is advisable to perform weekly blood monitoring during the first 18 weeks of therapy in patients taking olanzapine, and monthly thereafter[18].
The drug should be prescribed with caution to patients with a low number of leukocytes and/or neutrophils due to various reasons; with signs of suppression/toxic impairment of bone marrow function under the influence of drugs in the anamnesis; with suppression of bone marrow function due to concomitant disease, radiotherapy or chemotherapy in history; with hypereosinophilia or myeloproliferative disease. In clinical studies, the use of olanzapine in patients with a history of clozapine-dependent neutropenia or agranulocytosis was not accompanied by relapses of these disorders.
When prescribing olanzapine for the first time, it is necessary to assess the patient’s likelihood of weight gain, taking into account his body mass index, medical history, general clinical feeling of a tendency to be overweight - pasty, loose. When observing a patient taking olanzapine, it is important to consider the basic principle of weight gain control: a seven percent increase in body weight from the initial one is an absolute contraindication to further use of the drug[6].
To prevent obesity and its complications
(in particular, diabetes) it is necessary:
- Monitor body weight[19][20] and body mass index[20], fasting glucose (or hemoglobin A1c[21]) and plasma lipid levels[4][19][ 20]. Fasting glucose levels should not exceed 126 mg/dl, hemoglobin A1c should not exceed 6.1%[21]. To detect hyperglycemia, it is also advisable to measure not only the fasting glucose level, but also the level 2 hours after taking glucose [22]. In patients with risk factors (family history, excess weight), glucose levels should be monitored every 2–4 months[21]. It is also recommended to measure blood pressure in all patients before starting therapy and during therapy[20].
- The dose of the antipsychotic is increased slowly, which helps partially prevent weight gain. The first weeks of antipsychotic therapy are especially important, since it is much easier to prevent weight gain than to reduce it in the future [23].
- Be attentive to the patient’s lifestyle and diet. It is necessary that the diet be as low in calories as possible, and that the lifestyle be as active as possible. At the same time, diet and physical activity require careful dosing[14]. It is recommended to reduce the intake of saturated fat and cholesterol and increase the intake of fibrous foods. Smoking cessation is also recommended[24].
- If significant weight gain is noticed, refer the patient to a nutritionist and physical therapy specialist[4].
- When taking high doses of an antipsychotic, a careful approach to combining it with other diabetogenic drugs (beta-blockers, glucocorticoids, protease inhibitors, thiazide diuretics)[19].
To prevent the development of life-threatening conditions associated with diabetes (acidosis and coma), it is necessary to recognize and treat developing diabetes early. Psychiatrists when treating olanzapine should be alert to symptoms of diabetes such as weight loss, drowsiness, thirst, polyuria,[19] and, if necessary, provide their patient with consultation with an endocrinologist.[21]
When using olanzapine, the development of neuroleptic malignant syndrome is possible - a potentially fatal symptom complex, the clinical manifestations of which include a significant increase in body temperature, muscle rigidity, changes in mental status and autonomic disorders (unstable pulse or blood pressure, tachycardia, cardiac arrhythmia, increased sweating). Additional features may include increased CPK levels, myoglobinuria (rhabdomyolysis), and acute renal failure. Clinical manifestations of neuroleptic malignant syndrome or a significant increase in body temperature without other symptoms of this syndrome require discontinuation of all antipsychotics, including olanzapine.
In comparative studies lasting more than 6 weeks, treatment with olanzapine was significantly less likely to be accompanied by the development of tardive dyskinesia (an irreversible neurological side effect) than the use of haloperidol. However, the risk of this side effect should still be taken into account during long-term therapy with antipsychotics. If signs of tardive dyskinesia develop, it is recommended to reduce the dose or discontinue olanzapine. Symptoms of tardive dyskinesia may increase or manifest after discontinuation of the drug.
The drug should be used with extreme caution when the activity of AST and ALT increases in patients with insufficiency of liver function, limited functional reserve of the liver, or in patients receiving treatment with potentially hepatotoxic drugs. If the activity of AST and/or ALT increases during treatment with olanzapine, careful monitoring of the patient is required and, if necessary, dose reduction.
Olanzapine should be used with caution in patients with a history of epileptic seizures or exposed to factors that lower the seizure threshold. In such patients, seizures were rarely observed during olanzapine treatment.
In clinical studies, olanzapine therapy was rarely accompanied by side effects associated with the anticholinergic activity of the drug. However, clinical experience with olanzapine in patients with concomitant diseases is limited, so caution is recommended when prescribing olanzapine to patients with clinically significant prostatic hypertrophy, paralytic ileus, angle-closure glaucoma and similar conditions.
In vitro
Olanzapine exhibits dopamine antagonism and, like other antipsychotics, inhibits the effects of levodopa and dopamine agonists.
Given the nature of the action of olanzapine on the central nervous system, it should be used with caution in combination with other centrally acting drugs and ethanol.
Withdrawal from olanzapine may result in cholinergic withdrawal effects[25], including flu-like symptoms, insomnia, agitation, confusion[26], restlessness, anxiety, and extrapyramidal disturbances. To prevent the cholinergic effects of withdrawal, it is recommended to gradually reduce the dose of the drug (and, if a transfer to another antipsychotic is planned, a gradual increase in the dose of this antipsychotic), with the development of symptoms about, to the previous dose of the drug being discontinued, and its slower withdrawal, if necessary, the appointment of correctors and benzodiazepines[25].
Use in pediatrics
The safety and effectiveness of olanzapine in patients under 18 years of age have not been studied.
Impact on the ability to drive vehicles and operate machinery
Patients taking this drug should use caution when operating mechanical vehicles, including a vehicle, as olanzapine may cause drowsiness.
Olanzapine
Currently, many researchers consider olanzapine (Zyprexa) to be the most effective and safe drug for the treatment of schizophrenia. This medicine appeared on the pharmaceutical market in 1996.
Chemical group: thienobenzodiazepine.
Release form: tablets 2.5, 5, 7.5, 10, 15, and 20 mg; Available in soluble form (Zidis) in tablets of 5, 10, 15 and 20 mg, solutions for injection - 10 mg.
Pharmacokinetics: half-life - 31 hours, peak concentration - 6 hours, concentration stabilization is achieved within 7 days, bioavailability - 60%.
Dosage regimen: the dose range for adults ranges from 5 to 20 mg, but in practice, unfortunately, the maximum permissible doses of the drug often exceed the recommended level. For children, olanzapine is prescribed at a rate of 0.12 - 0.2 mg per kg of body weight, usually 1-3 times a day; for elderly people, the recommended dose range is 2.5-10 mg per day.
Indications: Olanzapine leads to a significant reduction in psychopathological symptoms of schizophrenia and is taken by patients longer than others without refusal if long-term administration is necessary (Lieberman J. et al., 2005).
According to some researchers, a clearly expressed antipsychotic effect of olanzapine is observed (after 2 - 3 weeks) primarily in patients with paroxysmal-progressive schizophrenia with acute paranoid symptoms (Eshimbetova S.Z., 2005). The therapeutic effect of an acute episode of schizophrenia during treatment with olanzapine is 76-80% (Morozova M.A. et al., 2000), and the overall reduction of psychosis is ahead of the reduction in the severity of delusions and hallucinations (Morozova M.A., Zharkova N.B., Beniashvili A.G., 2000).
When switching from typical antipsychotics to intramuscular injections of olanzapine, in paranoid schizophrenia, there are indications in the literature of a possible exacerbation of the condition. Due to the above, in this case, a gradual transition from one drug to another is desirable. Short-term prescription of benzodiazepine tranquilizers is possible. After olanzapine injections, patients become more accessible to communicate with medical personnel.
According to some authors (Kozyrev V.N. et al., 2005), hallucinatory-delusional symptoms with intramuscular administration of olanzapine are reduced much more slowly than agitation, angry-intense affect, anxiety and fear.
Treatment of schizophrenia with olanzapine allows, within two weeks, to reduce the severity of general psychopathological symptoms in this disease by 52.46%, productive symptoms by 47.73%, and negative symptoms by 13.33%. The parameters of neuropsychological disorders significantly improve: verbal memory by 13%, visual memory by 10%, working memory and attention by 5%, executive function by 1%. With further therapy for 4 weeks, negative symptoms (by 46.67%) and, to a lesser extent, positive symptoms (25.05%) are significantly reduced; indicators of verbal memory (by 10%) and working memory (by 8%) continue to improve. , indicators of visual memory and executive functions significantly increase (by 2%). (Panina A.N., Govorin N.V., 2005).
Studies have shown that cognitive impairment in patients with schizophrenia is associated with psychopathological disorders of the positive and negative spectrum, and their reduction during treatment with olanzapine occurs unevenly.
In the treatment of resistant schizophrenia, a more positive effect of olanzapine (4-6 weeks of treatment) was observed than of clozapine (Oleneva E.V., Tsukarzi E.E., Mosolov S.N., 2005).
The drug, compared to clozapine and risperidone, has a greater ability to eliminate symptoms of depression in schizophrenia, thereby reducing the risk of suicide (Meltzer H., 1999).
Mechanism of action: olanzapine has pronounced antagonism towards 5HT2A, 5HT2C (weight gain?), 5HT3, 5HT6 receptors and less pronounced antagonism towards D2 receptors (3: 1) (D2/D3 > D1/D3/D4). In addition, this drug has noticeable sensitivity to M1 and M5 (anticholinergic side effects), moderate tropism to H1 (somnolence) receptors and has little effect on alpha1-adrenergic receptors.
In terms of the spectrum of its pharmacological effect, olanzapine, of all the new generation antipsychotics, is closest to clozapine, but unlike the latter, it has a weaker effect on serotonergic, alpha2-adrenergic and cholinergic receptors. Olanzapine, like clozapine, has a higher affinity for HT2A receptors than for dopamine D2 receptors.
Indications for treatment of schizophrenia with olanzapine
- Pronounced manifestations of positive symptoms, especially acute and chronic hallucinatory-paranoid syndromes
- Psychomotor agitation
- Affective disorders of the depressive (anxiety, dysphoria) and manic spectrum with bipolar mania and bipolar depression
- Pronounced manifestations of negative symptoms
- Persistent manifestations of neurocognitive deficits
- Resistant forms of schizophrenia
- Suicidal tendencies
Olanzapine (Zyprexa) is prescribed in a dose of 2.5-10 mg once orally at night. On the second day of therapy, the dose can be increased to 15 mg. If there is no effect within two to three weeks, the dose is increased to 20 mg per day. The average dose of olanzapine in the treatment of schizophrenia is 5-20 mg, the average maintenance dose is 12.5 mg
The duration of treatment with olanzapine when administered intramuscularly ranges from 1 to 7 days (average 3 days). The first injection of the drug is prescribed at a dose of 10 mg, the second after 2 hours, the third, if necessary, after 4 hours (maximum daily dose 30 mg). In case of pathology of the liver and kidneys, with a combination of factors that reduce the half-life of the drug (elderly women, non-smokers), the number of injections is limited to 1-2 (Kozyrev V.N. et al., 2005).
Conversion from intramuscular injections to oral administration is recommended at a dose of the latter equal to 10-20 mg per day.
In addition to taking the drug in tablet form, an intramuscular form of olanzapine is used, which has sedative properties and has been proposed to relieve psychomotor agitation in schizophrenia.
Parenteral administration of drugs in the treatment of schizophrenia is indicated for the rapid relief of psychomotor agitation, unsatisfactory cooperation between the patient and the doctor, and in the presence of gastroenterological diseases.
When olanzapine is administered intramuscularly, its maximum plasma concentration is reached after 15-45 minutes, while when administered orally - after 3-6 hours (the half-life of the drug is 33 hours, the change in plasma concentration is proportional to the change in dose). 15, 30 and 45 minutes after the first administration, olanzapine appears to be more effective in terms of its impact on psychopathological symptoms than haloperidol (Wright P. et al., 2001).
Side effects: Side effects of olanzapine include drowsiness, dizziness, and weight gain. There are works in the literature indicating the ability of the drug to cause agranulocytosis, and rare cases of rhabdomyolysis have been noted.
Side effects of olanzapine
- Metabolic disorders (impaired fat and carbohydrate metabolism)
- Rarely cardiovascular disorders: orthostatic hypotension, bradycardia, edema, syncope
- Rarely gastroenterological disorders (nausea)
- Rarely, epileptiform syndrome and extrapyramidal symptoms (akathisia when using high doses of the drug)
- Rarely, rhabdomyolysis and agranulocytosis
- Slight and short-term increase in prolactin
Weight gain with olanzapine is more pronounced than with other atypical antipsychotics. This complication of therapy is difficult for patients, especially women, and can lead to not only somatic, but also neurotic disorders. Prevention of this complication is a low-calorie diet and thoughtful exercise programs.
The diet consists of reduced portions of food intake, preference for vegetables and fruits, reduction of high-calorie foods to a minimum and intake of at least 8 glasses of water daily.
At various times, drugs such as nizatidine, amantadine, topiramate, sibutramine, etc. were proposed for the treatment of this complication. However, the effect of taking these drugs and the mechanism of their action in relation to weight loss in patients in most studies turned out to be weak.
Attempts are currently being made to limit olanzapine-induced weight gain with bupropion (150-300 mg), an antidepressant with noradrenergic and dopaminergic effects. Bupropion is a relatively weak norepinephrine reuptake inhibitor and has virtually no effect on central serotonergic neurons (Gadde K. et al., 2006).
Bupropion can be taken with olanzapine without negative interactions between the two drugs. However, some caution is required when taking bupropion in terms of the occurrence of manic syndrome in persons with a history of its manifestations. In addition, taking bupropion promotes a faster reduction of the negative symptoms of schizophrenia.
It has been noted that a 24-week course of therapy with this antidepressant, on average, reduces weight over this period by almost 3 kg, and a decrease in blood cholesterol levels is also recorded, while the levels of cholesterol, lipoproteins, triglycerides and glucose in the blood remain virtually unchanged.
Initial weight loss persists for almost three months after stopping bupropion.
Body scans using X-ray absorptiometry (DXL) showed that weight loss during bupropion treatment was due to loss of trunk fat tissue. At the same time, weight loss is not accompanied by changes in bone density and mineral composition. Please note that if you have bulimia or anorexia, taking bupropion is contraindicated.
Less common side effects of olanzapine include nausea, orthostatic hypotension, bradycardia, edema, anticholinergic effects and extrapyramidal symptoms.
When taking olanzapine, an increase in the level of liver transaminases (AST and ALT) is possible, but it has practically no clinical significance. A reversible decrease in neutrophils and seizures are extremely rare. According to our observation, the latter complication occurs in 0.5% of cases and more often in children and adolescents.
Taking olanzapine can cause hyperlipedemia, an increase in the blood concentration of glycated hemoglobin, hyperinsulinemia and insulin resistance (Melkersson K. et al., 2000). During treatment with this drug, it is recommended to monitor the level of glucose and lipids in the blood, as well as monitor changes in the density of bone minerals in the body.
Prolactinemia is rare when taking olanzapine.
Contraindications to taking olanzapine: leukopenia, glaucoma, prostate hyperplasia. Caution is required when treating patients with liver diseases and relieving intoxication psychoses.
Interaction with medications: when co-administered with benzodiazepines, orthostatic hypotension and syncope may occur. Carbamazepine reduces the plasma concentration of olanzapine; Fluvoxamine, cimetidine and smoking, on the contrary, reduce it.
Return to Contents
Overdose
Symptoms.
Very often (>=10%) - tachycardia, agitation/aggression, articulation disorder, various extrapyramidal disorders and disturbances of consciousness of varying severity (from sedation to coma). Other clinically significant effects of olanzapine overdose include delirium, seizures, neuroleptic malignant syndrome, respiratory depression, aspiration, hypertension or hypotension, cardiac arrhythmias (<2% of overdose cases), and cardiac and respiratory arrest.
The minimum dose for an acute overdose with a fatal outcome was 450 mg, the maximum dose for an overdose with a favorable outcome (survival) was 1500 mg.
Treatment.
There is no specific antidote for olanzapine. Artificially inducing vomiting is not recommended. Standard detoxification methods are shown (gastric lavage, taking activated charcoal). Concomitant use of activated charcoal reduces the bioavailability of olanzapine when taken orally by 50-60%.
Symptomatic treatment is indicated in accordance with the clinical condition and monitoring of vital organ functions, including treatment of arterial hypotension, vascular collapse and support of respiratory function. You should not use adrenaline, dopamine and other sympathomimetics, which are b-adrenergic receptor agonists, since stimulation of the latter can aggravate arterial hypotension.
Olanzapine Canon, 28 pcs., 5 mg, film-coated tablets
Clinical improvement may take several days and requires patient monitoring. Neuroleptic malignant syndrome Neuroleptic malignant syndrome (NMS) (a potentially lethal symptom complex) can develop during treatment with any antipsychotics, including the drug Olanzapine Canon, but to date there is no data confirming a reliable connection between taking olanzapine and the development of this condition. Clinical manifestations of neuroleptic malignant syndrome include significant increase in body temperature, muscle rigidity, changes in mental status and autonomic disturbances (unstable pulse or blood pressure, tachycardia, cardiac arrhythmias, increased sweating). Additional features may include increased creatinine phosphokinase levels, myoglobinuria (rhabdomyolysis), and acute renal failure. Clinical manifestations of neuroleptic malignant syndrome or a significant increase in body temperature without other symptoms of neuroleptic malignant syndrome require discontinuation of all antipsychotics, including the drug Olanzapine Canon. Tardive dyskinesia Treatment with olanzapine is less often accompanied by the development of dyskinesia requiring drug correction than with haloperidol. However, the risk of tardive dyskinesia should be taken into account during long-term therapy with antipsychotics. If signs of tardive dyskinesia develop, it is recommended to reduce the dose or discontinue Olanzapine Canon. It should be taken into account that when switching to the drug Olanzapine Canon, symptoms of tardive dyskinesia may develop as a result of immediate withdrawal of previous therapy. Symptoms of tardive dyskinesia may increase or manifest after discontinuation of the drug. Parkinson's disease Efficacy when used in Parkinson's disease does not exceed placebo (for the purpose of relieving iatrogenic psychoses). The use of the drug Olanzapine Canon is not recommended in the treatment of psychoses induced by taking dopamine receptor agonists in Parkinson's disease; such patients experience increased symptoms of parkinsonism and hallucinations. Experience in elderly patients with psychosis due to dementia Cerebrovascular adverse events (eg, stroke, transient ischemic attack), including deaths, have been reported in elderly patients with psychosis due to dementia. In placebo-controlled studies, a higher incidence of cerebrovascular adverse events was observed in patients in the olanzapine group compared with the placebo group. These patients had previous risk factors (cerebrovascular disorders (history), transient ischemic attack, arterial hypertension, smoking), as well as concomitant diseases and/or medications that were time-related. with cerebrovascular disorders. The effectiveness of olanzapine in elderly patients with psychosis due to dementia has not been established. The main risk factors for increased mortality in this group of patients when treated with olanzapine are age > 80 years, sedation, concomitant use with benzodiazepines, or the presence of pulmonary pathology (eg, pneumonia with or without aspiration). There are insufficient data to establish differences in the incidence of cerebrovascular events and/or mortality (compared with placebo) and risk factors in this group of patients between oral and intramuscular olanzapine. The drug Olanzapine Canon is not recommended for the treatment of patients with psychosis due to dementia. Development of the risk of sudden death Clinical experience with all antipsychotics, including olanzapine, has shown a similar dose-dependent twofold increase in the risk of death due to acute heart failure compared with deaths due to acute heart failure in patients not taking antipsychotics. QT interval duration: Clinically significant increases in the QT interval have been reported infrequently in patients receiving olanzapine, with no significant differences from placebo in the incidence of cardiac adverse events. However, as with the use of other antipsychotic drugs, it is recommended to exercise caution when prescribing Olanzapine Canon in combination with drugs that can prolong the QT interval, especially in elderly patients, with congenital prolongation of the QT interval, with congestive heart failure, myocardial hypertrophy, hypokalemia and hypomagnesemia. Postural hypotension Postural hypotension is uncommon in elderly patients. Just as when using other antipsychotics, when Olanzapine Canon is prescribed to patients over 65 years of age, it is recommended to monitor blood pressure. Thromboembolism The development of venous thromboembolism is extremely rare during olanzapine therapy. The presence of a cause-and-effect relationship between taking olanzapine and venous thromboembolism has not been established. However, given that patients with schizophrenia often have acquired risk factors for venous thromboembolism, it is necessary to conduct a cumulative assessment of all possible risk factors for the development of this complication, including immobilization of patients, and take the necessary preventive measures. Liver dysfunction In some cases, taking olanzapine, usually in the early stages of therapy, is accompanied by a transient, asymptomatic increase in liver transaminases (AST and ALT) in the blood serum. Rare cases of hepatitis have been reported. In very rare cases, hepatic cholestasis and other mixed liver damage have been observed. Particular caution is required when serum AST and/or ALT levels increase in patients with impaired liver function, with limited liver functional reserve, or in patients receiving treatment with potentially hepatotoxic drugs. If AST and/or ALT levels increase during treatment with olanzapine, careful monitoring of the patient is required and, if necessary, a reduction in the dose of Olanzapine Canon. If hepatitis, including hepatocellular, cholestatic or mixed, is detected, Olanzapine Canon should be discontinued. Hyperglycemia and diabetes mellitus There is a higher prevalence of diabetes mellitus in patients with schizophrenia. As with some other antipsychotic drugs, very rare cases of hyperglycemia, diabetes mellitus, exacerbation of pre-existing diabetes mellitus, ketoacidosis and diabetic coma have been reported. A cause-and-effect relationship between antipsychotic drugs and these conditions has not been established. Close clinical monitoring of patients with diabetes mellitus and patients with risk factors for developing diabetes mellitus is recommended. For all groups of patients, regardless of body mass index, a clinically significant increase in body weight was observed. An increase of 7% or more from the mean after a short course of treatment (average duration of 47 days) was very common (22.2%), an increase of 15% or more was common (4.2%), and an increase of 25% or more was infrequent (0.8%). In patients receiving longer treatment (at least 48 weeks), increases of >7%, >15% and >25% were very common (64.4%, 31.7%, 12.3%, respectively). Changes in lipid profile Undesirable changes in lipid profile have been observed in patients treated with olanzapine. Monitoring of the lipid profile and clinical observation are recommended. Epileptic seizures Olanzapine Canon should be used with caution in patients with a history of epileptic seizures or those exposed to factors that lower the seizure threshold. In these patients, seizures are rare when treated with olanzapine. Hematological changes As with other antipsychotics, caution should be exercised when treating patients with olanzapine with a low number of leukocytes and/or neutrophils in the peripheral blood due to various reasons; with signs of suppression or toxic impairment of bone marrow function under the influence of drugs in the anamnesis; with suppression of bone marrow function due to concomitant disease, radiotherapy or chemotherapy in history; with hypereosinophilia or myeloproliferative disease. In clinical studies, the use of olanzapine in patients with a history of clozapine-dependent neutropenia or agranulocytosis was not accompanied by relapses of these disorders. The development of neutropenia has been reported primarily during concomitant therapy with olanzapine and valproic acid. Anticholinergic activity Olanzapine therapy is rarely associated with anticholinergic side effects. However, clinical experience with olanzapine in patients with concomitant diseases is limited, so caution is recommended when prescribing Olanzapine Canon to patients with clinically significant prostatic hypertrophy, paralytic ileus and similar conditions. Dopaminergic Antagonism In vitro, olanzapine exhibits dopamine receptor antagonism and, like other antipsychotics, may theoretically inhibit the effects of levodopa and dopamine agonists. General activity in relation to the central nervous system. Given the primary CNS effects of olanzapine, caution should be exercised when using olanzapine in combination with other centrally acting drugs and alcohol. Suicide The risk of suicide attempts in patients with schizophrenia and bipolar disorder type 1 is due to the diseases themselves. In this regard, during pharmacotherapy, careful monitoring of those patients whose risk of suicide is especially high is required. When prescribing Olanzapine Canon, one should strive to minimize the number of tablets taken by the patient in order to reduce the risk of overdose. Withdrawal of therapy In case of abrupt withdrawal of olanzapine, sweating, insomnia, tremor, nausea and vomiting rarely (0.01 - 0.1%) develop. When discontinuing the drug, a gradual dose reduction is recommended. Children and adolescents under 18 years of age Olanzapine is not recommended for use in children and adolescents under 18 years of age due to the lack of sufficient data on efficacy and safety. In short-term studies that were conducted in adolescents 13-17 years of age, there was a greater increase in body weight and changes in lipid and prolactin concentrations than in similar studies in adults.
Effect on the ability to drive vehicles Patients taking the drug Olanzapine Canon should be careful when driving vehicles and work that requires rapid psychomotor reactions, since olanzapine can cause drowsiness and dizziness.
Drug interactions
The metabolism of olanzapine may be altered by inhibitors or inducers of cytochrome P450 isoenzymes that exhibit specific activity against CYP1A2. The clearance of olanzapine increases in smoking patients and in patients taking carbamazepine (due to increased CYP1A2 activity). Known potential inhibitors of CYP1A2 may reduce the clearance of olanzapine. Olanzapine is not a potential inhibitor of CYP1A2 activity, therefore the pharmacokinetics of drugs such as theophylline, which are metabolized primarily by CYP1A2, are not altered when taking olanzapine.
Clinical trials have shown that a single dose of olanzapine during therapy with the following drugs was not accompanied by suppression of their metabolism: imipramine or its metabolite desipramine (CYP2D6, CYP3A4, CYP1A2), warfarin (CYP2C19), theophylline (CYP1A2) or diazepam (CYP3A4, CYP2C19) . There were also no signs of drug interactions when olanzapine was used in combination with lithium or biperiden.
Against the background of steady-state concentrations of olanzapine, no changes in the pharmacokinetics of ethanol were observed. However, taking ethanol with olanzapine may be accompanied by increased pharmacological effects of olanzapine, such as sedation.
A single dose of an aluminum- and magnesium-containing antacid or cimetidine does not affect the bioavailability of olanzapine when taken orally. Simultaneous administration of activated carbon reduces the bioavailability of olanzapine by 50-60%.
Fluoxetine (60 mg once or 60 mg daily for 8 days) causes an average 16% increase in olanzapine Cmax and an average 16% decrease in olanzapine clearance. The degree of influence of fluoxetine is significantly lower than the severity of individual differences in these indicators, therefore it is usually not recommended to change the dose of olanzapine when prescribed in combination with fluoxetine.
in vitro studies
using human liver microsomes, it was shown that olanzapine slightly inhibits the formation of valproate glucuronide (the main pathway of valproate metabolism).
Valproate also has little effect on the in vitro
. Therefore, a clinically significant pharmacokinetic interaction between olanzapine and valproate is unlikely.
Based on in vitro
Using human liver microsomes, olanzapine has very little potential to inhibit the activity of the following cytochrome P450 isoenzymes: CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4.
Potentiates the effect of drugs that depress the central nervous system: neuroleptics, tranquilizers, antidepressants, anticonvulsants, mood stabilizers, ethanol.
Olanzapin-Teva in Moscow
pharmachologic effect
Olanzapine is an antipsychotic drug (neuroleptic) with a wide pharmacological spectrum of effects on a number of receptor systems. The antipsychotic effect is due to antagonism towards 5HT2A/2C-; 5HT3-, 5HT6-serotonin receptors, D1-, D2-, D3-, D4-, D5-dopamine receptors, m-anticholinergic effects - blockade of M1-5 - muscarinic cholinergic receptors; also has an affinity for alpha1-adrenergic and H1-histamine receptors. In vivo and in vitro, olanzapine has a more pronounced affinity and activity for 5HT2-serotonin receptors compared to D2-dopamine receptors.
Olanzapine selectively reduces the excitability of mesolimbic (A10) dopaminergic neurons and has a slight effect on striatal (A9) nerve pathways involved in the regulation of motor functions. Olanzapine reduces the conditioned defense response (a test that measures antipsychotic activity) at lower doses than doses that cause catalepsy (a disorder that reflects an effect on motor function). Unlike other antipsychotics, olanzapine enhances the anti-anxiety effect when performing the “anxiolytic” test.
Olanzapine reduces the delta rhythm (1-4 Hz) in the anterior parts of the fronto-central regions of the brain (F3.4, C3.4), diffusely enhances the theta range (4-8 Hz) in the same fronto-central and parietal-central regions areas, and also enhances the alpha rhythm (8-13 Hz) in the occipital and parietal cortical areas. An increase in the alpha rhythm indicates a normalization of the EEG structure under the influence of olanzapine, which produces a global inhibitory effect in almost all parts of the brain, with the exception of the frontal regions.
Eliminates the productive symptoms of psychosis (delusions, hallucinations, thinking disorders, hostility, suspicion), reduces negative symptoms (emotional and social autism, introversion, poor speech). Dulls the severity of emotional experiences, weakens aggressiveness and impulsiveness of behavioral reactions, forms tolerance to the surrounding reality and reduces initiative. Relieves agitation and corrects behavioral and mental disorders in patients with mental disorders.
Pharmacokinetics
After oral administration, olanzapine is well absorbed from the gastrointestinal tract. Food intake does not affect the bioavailability of olanzapine. Bioavailability is reduced by 40% due to the “first pass” effect through the liver. Cmax in blood plasma is achieved after 5-8 hours. Equilibrium concentration is achieved after 1 week of daily administration and is twice the plasma concentration after a single dose. Plasma concentration in the dose range from 1-20 mg changes linearly and is proportional to the dose.
At plasma concentrations from 7 to 1000 ng/ml, binding to plasma proteins, mainly albumin and alpha1-acid glycoprotein, is about 93%.
Passes through histohematic barriers, including the blood-brain barrier. Distribution volume approx. 1000 l.
Olanzapine is metabolized in the liver by conjugation and oxidation. The main circulating metabolite is 10-N-glucuronide, which theoretically does not cross the blood-brain barrier. Cytochrome P450 isoenzymes CYP1A2 and CYP2D6 are involved in the formation of N-desmethyl and 2-hydroxymethyl metabolites of olanzapine. The main pharmacological activity of the drug is due to the parent substance - olanzapine. Metabolites have significantly less pronounced pharmacological activity in vivo than olanzapine. The activity of the cytochrome P450 isoenzyme CYP2D6 does not affect the rate of olanzapine metabolism. About 57% of an oral dose of olanzapine is excreted in the urine, mainly in the form of metabolites.
T1/2 and clearance of olanzapine (KO) vary depending on gender, age, and the presence of smoking habits. In young healthy volunteers (mixed population), T1/2 averages 33 hours (21-54 hours), and the average total plasma CO is 26 l/h (12-47 l/h). In healthy elderly volunteers (age 65 years and older), T1/2 is extended to 51.8 hours, CO is reduced to 17.7 l/hour. In women, compared to men, T1/2 of olanzapine is higher (36.7 hours versus 32.3 hours), and CO is lower (18.9 ml versus 27.3 l/h). In non-smoking men and women, compared to smokers, T1/2 increases (38.6 hours versus 30.4 hours), and CO decreases (18.6 l/h versus 27.7 l/h). However, the degree of changes in T1/2 and total plasma CR depending on gender, age, and smoking habits is significantly inferior to the degree of individual differences in these indicators.
There were no significant differences between the average T1/2 and CR values in patients with severely impaired renal function compared with individuals with normal renal function.
In smoking patients with minor liver dysfunction, T1/2 is higher (48.8 hours) and CO is lower (14.1 l/h) than in non-smokers without liver dysfunction (T1/2 - 39.3 hours, CO - 18 l/h).
In persons over 65 years of age, the T1/2 half of olanzapine may be significantly prolonged, so the average daily dose of olanzapine should be lower than usual. In studies involving subjects of European, Japanese and Chinese populations, differences in the pharmacokinetics of olanzapine associated with race were not established.
Indications
— schizophrenia: treatment of exacerbations, maintenance and long-term anti-relapse therapy;
- bipolar affective disorder: treatment of acute manic or mixed episodes;
- to prevent relapses in patients with bipolar affective disorder in whom olanzapine was effective in treating the manic phase.
Dosage regimen
Inside, regardless of food intake.
For schizophrenia
The recommended starting dose is 10 mg once a day.
For the treatment of an acute manic episode in bipolar disorder
The recommended initial dose is 15 mg once a day (when used as monotherapy) or 10 mg once a day (when used in combination with lithium or valproic acid).
To prevent relapses of bipolar affective disorder
The recommended starting dose is 10 mg once a day.
In the treatment of schizophrenia, an acute manic episode in bipolar affective disorder and for the prevention of relapses of bipolar affective disorder, doses of olanzapine are selected individually depending on the clinical status of the patient and vary in the range of 5-20 mg 1 time per day. Increasing the dose above the standard dose (15 mg 1 time per day) is recommended only after an appropriate clinical examination of the patient. The dose should be increased gradually, at intervals of at least 24 hours.
A reduction in the initial dose is recommended in patients with a combination of factors (female patients, elderly patients, non-smokers) that may slow the metabolism of olanzapine.
Elderly patients
, as well as in case of
severe renal failure
or
moderate liver failure,
the drug is used in an initial dose of 5 mg 1 time per day.
Side effect
The frequency of side effects is classified in accordance with the recommendations of the World Health Organization: very often - at least 10%; often - at least 1%, but less than 10%; infrequently - not less than 0.1%, but less than 1%: rarely - not less than 0.01%, but less than 0.1%: very rarely - not less than 0.01%, including isolated messages.
From the blood and lymphatic system:
often - eosinophilia; rarely - leukopenia; very rarely - thrombocytopenia, neutropenia.
From the side of metabolism:
very often - weight gain; often - increased appetite; unknown frequency - development or exacerbation of diabetes mellitus, diabetic ketoacidosis, diabetic coma, including death.
From the nervous system:
very often - drowsiness; often - dizziness, akathisia, parkinsonism, dyskinesia, gait disturbance (in patients with Alzheimer's type dementia); rarely - extrapyramidal disorders (mainly when using high doses); very rarely - sweating, insomnia, tremor, anxiety, nausea; unknown frequency - neuroleptic malignant syndrome (NMS), dystonia (including oculogyric crisis), tardive dyskinesia.
From the cardiovascular system:
often - orthostatic hypotension; infrequently - bradycardia, prolongation of the QT interval; unknown frequency - ventricular tachycardia/ventricular fibrillation; sudden death, pulmonary embolism, deep vein thrombosis.
From the digestive system:
often - dryness of the oral mucosa, constipation (m-anticholinergic effect); very rarely - hepatitis (including hepatocellular, cholestatic or mixed), pancreatitis.
For the skin and subcutaneous tissues:
infrequently - photosensitivity reaction; rarely - skin rash; very rarely - alopecia.
From the musculoskeletal system:
very rarely - rhabdomyolysis.
From the genitourinary system:
infrequently - urinary incontinence; very rarely - priapism, urinary retention.
Laboratory indicators:
very often - an increase in the concentration of prolactin in the blood plasma *; often - increased concentrations of glucose, cholesterol and triglycerides in the blood plasma, glucosuria, transient increase in the activity of liver enzymes (aspartate aminotransferase (ACT) and alanine aminotransferase (ALT)); uncommon - increased activity of creatine phosphokinase (CPK); very rarely - increased alkaline phosphatase activity and total bilirubin concentration.
* The increase in prolactin concentration in blood plasma is mild and transient (the average value of maximum prolactin concentrations did not reach the upper limit of normal and was not statistically significantly different from placebo). Clinical manifestations of hyperprolactinemia possibly related to olanzapine (i.e., amenorrhea, galactorrhea, breast enlargement in women, gynecomastia in men) have been reported rarely. Sexual dysfunction possibly related to the use of olanzapine (erectile dysfunction in men, decreased libido in men and women) was frequently observed. In most patients, normalization of prolactin concentrations was observed without discontinuation of olanzapine.
Other:
often - asthenia, fatigue, peripheral edema; unknown frequency - hypothermia, withdrawal syndrome (increased sweating, insomnia, tremor, anxiety, nausea, vomiting).
Special patient groups
In older patients with dementia-related psychosis:
very often - cerebrovascular disorders (stroke, transient ischemic attacks), including fatal ones, gait disturbance and falls; often - urinary incontinence and pneumonia.
In patients with psychosis induced by a drug (dopamine receptor agonist) for the treatment of Parkinson's disease:
very often - increased symptoms of parkinsonism and hallucinations.
In patients with bipolar mania taking olanzapine in combination with lithium or valproic acid:
very often - weight gain, dry oral mucosa, increased appetite, tremor; often - speech disorder.
Contraindications for use
- history of angle-closure glaucoma;
- period of breastfeeding;
- age under 18 years (efficacy and safety have not been established);
- lactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome;
- hypersensitivity to olanzapine and other components of the drug.
Carefully:
Kidney failure; liver failure; in patients receiving treatment with potentially hepatotoxic drugs; benign prostatic hyperplasia; neutropenia; myelosuppression (including due to concomitant diseases, chemotherapy and radiation therapy); myeloproliferative diseases; hypereosinophilic syndrome; conditions predisposing to the development of arterial hypotension (dehydration, hypovolemia, taking antihypertensive drugs); cardiovascular diseases (myocardial infarction, coronary heart disease, heart failure, intracardiac conduction disorders, etc.); history of epileptic seizures; elderly patients (over 65 years), including those with dementia associated with psychosis and/or behavioral disorders; paralytic ileus and similar conditions; pneumonia; simultaneous use with centrally acting drugs, benzodiazepines, ethanol.
Use during pregnancy and breastfeeding
Women should be informed of the need to inform their doctor about an existing or planned pregnancy during therapy with Olanzapine-Teva.
Due to limited experience with the use of olanzapine during pregnancy, Olanzapine-Teva should be used to treat pregnant women only when the potential benefit of therapy to the mother outweighs the potential risk to the fetus.
Olanzapine is excreted in breast milk. If it is necessary to use the drug Olanzapine-Teva, breastfeeding should be discontinued.
Use in children
Contraindicated for use in patients under 18 years of age (efficacy and safety have not been established).
Overdose
Symptoms:
tachycardia, agitation/aggression, dysarthria, various extrapyramidal disorders and disturbances of consciousness of varying severity (from sedation to coma), delirium, convulsions, NMS, respiratory depression, aspiration, arterial hypertension or hypotension, ventricular tachycardia (less than 2% of overdose cases) , cardiac and respiratory arrest. The minimum dose for an acute overdose with a fatal outcome was 450 mg, the maximum dose for an overdose with a favorable outcome (survival) was 1500 mg.
Treatment:
there is no specific antidote. Artificially inducing vomiting is not recommended. Standard detoxification techniques are indicated (i.e., gastric lavage, activated charcoal). Concomitant use of activated charcoal reduces the bioavailability of olanzapine taken orally by 50-60%. Symptomatic treatment is carried out in accordance with the clinical condition and monitoring the functions of vital organs, including correction of arterial hypotension, vascular collapse and support of respiratory function. Epinephrine, dopamine and other sympathomimetics, which are beta-adrenergic receptor agonists, should not be used, because stimulation of the latter can aggravate arterial hypotension.
Drug interactions
The metabolism of olanzapine may be altered by inhibitors or inducers of cytochrome P450 isoenzymes that exhibit specific activity against the CYP1A2 isoenzyme. KO increases in smoking patients and in patients taking carbamazepine (due to increased activity of the CYP1A2 isoenzyme). Known potential inhibitors of the CYP1A2 isoenzyme can reduce CR. Olanzapine is not a potential inhibitor of the activity of the CYP1A2 isoenzyme, therefore, when taking olanzapine, the pharmacokinetics of drugs such as theophylline, which are metabolized mainly with the participation of the CYP1A2 isoenzyme, do not change.
Fluvoxamine, a specific inhibitor of the CYP1A2 isoenzyme, significantly alters the pharmacokinetics of olanzapine, increasing its Cmax by 54% in non-smoking women and by 77% in smoking men, with an increase in the area under the pharmacokinetic curve by 52% and 108%, respectively. The dose of olanzapine should be reduced in patients taking fluvoxamine or other CYP1A2 inhibitors, such as ciprofloxacin.
A single dose of olanzapine during therapy with the following drugs: imipramine or its metabolite desipramine (CYP2D6, CYP3A4, CYP1A2 isoenzymes), warfarin (CYP2C19 isoenzyme), theophylline (CYP1A2 isoenzyme) or diazepam (CYP3A4, CYP2C19 isoenzymes) was not accompanied by suppression of their metabolism. There were also no signs of drug interactions when olanzapine was used concomitantly with lithium or biperiden.
Olanzapine has very little potential to inhibit the activity of the following cytochrome P450 isoenzymes: CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4. A single dose of an aluminum- and magnesium-containing antacid or cimetidine does not affect the bioavailability of olanzapine when taken orally. Simultaneous administration of activated carbon reduces the bioavailability of olanzapine by 50-60%.
Fluoxetine (60 mg once or 60 mg daily for 8 days) causes an average 16% increase in olanzapine Cmax and an average 16% decrease in CR. The degree of influence of fluoxetine is significantly inferior to the severity of individual differences in pharmacokinetic parameters, therefore it is usually not recommended to change the dose of olanzapine when used in combination with fluoxetine.
In in vitro studies using human liver microsomes, olanzapine was shown to slightly inhibit the formation of valproic acid glucuronide (the main metabolic pathway of valproic acid). Valproic acid also has little effect on the metabolism of olanzapine. Therefore, a clinically significant pharmacokinetic interaction between olanzapine and valproic acid is unlikely.
Against the background of steady-state concentrations of olanzapine, no changes in the pharmacokinetics of ethanol were observed. However, taking ethanol with olanzapine may be accompanied by increased pharmacological effects of olanzapine, such as sedation.
Caution should be exercised when using olanzapine in patients who drink alcohol or are taking drugs that may cause central nervous system depression.
Concomitant use of olanzapine with antiparkinsonian drugs in patients with dementia due to Parkinson's disease is not recommended.
As with other antipsychotics, olanzapine should be used with caution when coadministered with drugs that prolong the QT interval.
Conditions for dispensing from pharmacies
On prescription.
Storage conditions and periods
Store at a temperature not exceeding 25°C in a place protected from light. Keep out of the reach of children. Shelf life: 2 years. Do not take after the expiration date stated on the package.
Use for liver dysfunction
Use with caution in case of liver failure
Use for renal impairment
Use with caution in renal failure
Use in elderly patients
Use with caution in elderly patients (over 65 years of age), including those with dementia associated with psychosis and/or behavioral disorders.
In persons over 65 years of age, the T1/2 half of olanzapine may be significantly prolonged, so the average daily dose of olanzapine should be lower than usual.
special instructions
Neuroleptic malignant syndrome.
When using any antipsychotics, including olanzapine, the development of NMS is possible, the clinical manifestations of which include a significant increase in body temperature, muscle rigidity, changes in mental status and autonomic disorders (tachycardia, unstable pulse or blood pressure, cardiac arrhythmia, increased sweating). Additional signs may include increased serum CPK concentrations, myoglobinuria (a symptom of rhabdomyolysis), and acute renal failure. Clinical manifestations of neuroleptic malignant syndrome or a significant increase in body temperature without other symptoms of this syndrome require discontinuation of all antipsychotics, including olanzapine.
Parkinson's disease.
The use of Olanzapine-Teva is not recommended for the treatment of psychosis in Parkinson's disease caused by the use of dopamine receptor agonists, due to the fact that parkinsonian symptoms and hallucinations may increase. The effectiveness of olanzapine for the treatment of psychotic symptoms in this case is not superior to placebo.
Dementia-related psychoses and/or behavioral disorders.
Olanzapine-Teva is not recommended for use in elderly patients with psychosis associated with dementia and/or behavioral disorders, due to the fact that in this group of patients there has been an increase in reports of the risk of developing cerebrovascular disorders (stroke, transient ischemic attacks) and death. It was found that the high mortality rate was not related to the dose of olanzapine or the duration of olanzapine treatment. Risk factors that may predispose patients to increased mortality in this population include age over 65 years, dysphagia, sedation, malnutrition, dehydration, pulmonary disease (pneumonia with or without aspiration), or concomitant use of benzodiazepines. Additionally, it was revealed that all patients who had cerebrovascular disorders, both in the group of patients taking olanzapine and in the placebo group, suffered from vascular dementia or mixed type dementia. The effectiveness of olanzapine in this group of patients has not been determined.
Hyperglycemia and/or development or exacerbation of diabetes mellitus.
In some cases, when using olanzapine, hyperglycemia, diabetes mellitus, exacerbation of pre-existing diabetes, diabetic ketoacidosis and diabetic coma, including death, may develop. Increased patient weight has been reported to be a predisposing factor for the development of these side effects. When using the drug Olanzapine-Teva in patients with diabetes mellitus or with risk factors for developing diabetes mellitus, it is necessary to exercise caution and monitor the manifestation of signs of hyperglycemia (polydipsia, polyuria, increased appetite, weakness), as well as regularly monitor the patient’s body weight and plasma glucose concentration blood.
Change in lipid concentration.
Changes in plasma lipid concentrations during treatment with Olanzapine-Teva should be monitored in patients with dyslipidemia and in patients with risk factors for developing lipid metabolic disorders.
M-anticholinergic effect.
Olanzapine therapy may be accompanied by adverse reactions associated with the manifestation of the m-anticholinergic effect. Clinical experience with olanzapine in patients with underlying medical conditions is limited, and caution is advised when using olanzapine in patients with clinically significant benign prostatic hypertrophy, paralytic ileus, angle-closure glaucoma, and other similar conditions.
Liver function.
Transient asymptomatic increases in the activity of hepatic transaminases (ALT and AST) were most often observed at the beginning of treatment with olanzapine. Caution should be exercised in patients with initially elevated ALT and/or AST levels, in patients with liver failure, limited liver functional reserve, or in patients receiving treatment with potentially hepatotoxic drugs. In case of hepatitis development (including hepatocellular, cholestatic or mixed etiology), Olanzapine-Teva should be discontinued.
Neutropenia
. Caution should be used in patients with low white blood cell and/or neutrophil counts due to any cause, including medications that cause neutropenia, bone marrow suppression due to concomitant diseases, history of radiotherapy or chemotherapy, and hypereosinophilia or myeloproliferative disease. Neutropenia typically occurs with concomitant use of olanzapine and valproic acid. The use of olanzapine in patients with a history of clozapine-dependent neutropenia or agranulocytosis was not accompanied by relapses of these disorders.
Withdrawal syndrome
. If you abruptly stop taking Olanzapine-Teva, in some cases a condition may develop that is accompanied by acute symptoms: increased sweating, insomnia, tremor, anxiety, nausea and vomiting.
Prolongation of the QT interval.
In clinical studies, clinically significant prolongation of the QTc interval (Friederician-corrected QT interval; QTcF interval prolongation of at least 500 ms in patients with baseline QTcF less than 500 ms) was observed in patients receiving olanzapine compared with placebo groups, which did not associated with any cardiovascular effects. However, as with other antipsychotic drugs, when taking Olanzapine-Teva, caution should be exercised when used concomitantly with drugs that prolong the QT interval, especially in the elderly, in patients with congenital long QT syndrome, in congestive heart failure, myocardial hypertrophy, hypokalemia or hypomagnesemia. During treatment with olanzapine, periodic monitoring of the electrocardiogram should be performed.
Thromboembolism.
Isolated cases of VTE have been reported while taking olanzapine. A cause-and-effect relationship between VTE and olanzapine has not been established. However, since patients with schizophrenia, along with acquired risk factors for VTE, may have all other possible risk factors for VTE, such as prolonged immobilization, it is necessary to identify these risk factors and implement VTE preventive measures.
Convulsive syndrome.
Olanzapine-Teva should be used with caution in patients who have a history of seizures or risk factors that may lower the seizure threshold.
Tardive dyskinesia.
In a comparative study, treatment with olanzapine for less than 1 year was significantly less likely to be accompanied by the development of dyskinesia requiring drug treatment than treatment with haloperidol. However, the risk of developing tardive dyskinesia increases with longer use of olanzapine. If signs or symptoms of tardive dyskinesia appear, consider reducing the dose of Olanzapine-Teva or discontinuing it. Symptoms of tardive dyskinesia may temporarily increase or appear even after stopping the drug.
Orthostatic hypotension.
Due to the adrenergic blocking effect, olanzapine can cause orthostatic hypotension, accompanied by dizziness, tachycardia, and fainting during the initial dose selection process. Orthostatic hypotension most often occurs in elderly patients and when using other antipsychotics. The development of these phenomena can be minimized by more fractional dose titration and starting therapy with a minimum dose. When using Olanzapine-Teva, blood pressure should be monitored, especially in patients over 65 years of age. If patients experience severe orthostatic hypotension, they should be warned not to stand abruptly and without assistance.
Sudden death.
Clinical experience with all antipsychotics, including olanzapine, has shown a similar dose-dependent twofold increase in the risk of sudden death compared with sudden death in patients not using antipsychotics.
Effect on the central nervous system (CNS).
Given the nature of the drug's action on the central nervous system, olanzapine should be used with caution in combination with other centrally acting drugs and ethanol.
In vitro, olanzapine exhibits dopamine receptor antagonism and, like other antipsychotics, may theoretically inhibit the effects of levodopa and dopamine receptor agonists.
Impact on the ability to drive vehicles and operate machinery
Caution should be exercised when using the drug Olanzapine-Teva due to the possible development of adverse reactions that may negatively affect the ability to drive vehicles and perform potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Criticism
In the USA, lawsuits were filed against Eli Lilly and Company, the manufacturer of Zyprexa (olanzapine), in connection with advertising the drug for off-label use and in connection with the concealment of certain side effects (hyperglycemia, diabetes mellitus)[27]. Eli Lilly has been very successful in recommending olanzapine off-label for dementia and depression, especially in children and the elderly, despite severe side effects including heart failure, pneumonia, excess weight, and diabetes, among others. Eli Lilly shills attended lectures and conferences for doctors about olanzapine and asked prepared questions. Aware of the risk of weight gain in patients, the company nevertheless minimized the link between olanzapine and excess weight in a widely circulated video, “The Diabetes Myth,” which used studies of questionable quality and integrity and false reporting of side effects.[28]
The company paid out more than a billion dollars to settle olanzapine lawsuits.[27] Lawsuits have also been filed in connection with the side effects of Symbiax (English)Russian. - a drug that combines the side effects of olanzapine and fluoxetine[29].
In 2007, Eli Lilly still claimed that "...multiple studies have not found that Zyprexa causes diabetes," even though Zyprexa and similar drugs have had FDA warnings about hyperglycemia on their labels since 2003. According to Eli Lilly's own research, 30% of patients gained at least 10 kg in weight within a year of use. The company also hid the fact that, according to a 1999 study, blood sugar levels in patients taking olanzapine steadily increased over 3 years. Both psychiatrists and endocrinologists testified that olanzapine led to the development of diabetes in many more patients than other drugs.[28]
OLANZAPINE CANON TAB. P/P/O 10MG No. 28
Clinical improvement may take several days and requires patient monitoring.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome (NMS) (a potentially lethal symptom complex) can develop during treatment with any antipsychotics, including the drug Olanzapine Canon, but to date there is no data confirming a reliable connection between taking olanzapine and the development of this condition. Clinical manifestations of neuroleptic malignant syndrome include significant increase in body temperature, muscle rigidity, changes in mental status and autonomic disturbances (unstable pulse or blood pressure, tachycardia, cardiac arrhythmias, increased sweating). Additional features may include increased creatine phosphokinase levels, myoglobinuria (rhabdomyolysis), and acute renal failure. Clinical manifestations of neuroleptic malignant syndrome or a significant increase in body temperature without other symptoms of neuroleptic malignant syndrome require discontinuation of all antipsychotics, including the drug Olanzapine Canon.
Tardive dyskinesia
Treatment with olanzapine is less often accompanied by the development of dyskinesia, requiring drug correction, than with the use of haloperidol. However, the risk of tardive dyskinesia should be taken into account during long-term therapy with antipsychotics. If signs of tardive dyskinesia develop, it is recommended to reduce the dose or discontinue Olanzapine Canon. It should be taken into account that when switching to the drug Olanzapine Canon, symptoms of tardive dyskinesia may develop as a result of immediate withdrawal of previous therapy. Symptoms of tardive dyskinesia may increase or manifest after discontinuation of the drug.
Parkinson's disease
Efficacy when used in Parkinson's disease does not exceed placebo (for the purpose of relieving iatrogenic psychoses). The use of the drug Olanzapine Canon is not recommended in the treatment of psychoses induced by taking dopamine receptor agonists in Parkinson's disease; such patients experience increased symptoms of parkinsonism and hallucinations.
Experience of use in elderly patients with psychosis due to dementia
Cerebrovascular adverse events (eg, stroke, transient ischemic attack), including deaths, have been reported in elderly patients with psychosis associated with dementia. In placebo-controlled studies, there was a higher incidence of cerebrovascular adverse events in patients in the olanzapine group compared with the placebo group. These patients had previous risk factors (history of cerebrovascular disorders, transient ischemic attack, arterial hypertension, smoking), as well as concomitant diseases and/or medications that were temporally associated with cerebrovascular disorders.
The effectiveness of olanzapine in elderly patients with psychosis due to dementia has not been established. The main risk factors for increased mortality in this group of patients when treated with olanzapine are age 80 years, sedation, concomitant use with benzodiazepines, or the presence of pulmonary pathology (eg, pneumonia with or without aspiration). There are insufficient data to establish differences in the incidence of cerebrovascular events and/or mortality (compared with placebo) and risk factors between oral and intramuscular olanzapine in this group of patients. The drug Olanzapine Canon is not recommended for the treatment of patients with psychosis due to dementia.
Development of the risk of sudden death
Clinical experience with all antipsychotics, including olanzapine, has shown a similar dose-dependent two-fold increase in the risk of death due to acute heart failure compared with death due to acute heart failure in patients not taking antipsychotics.
Duration of the QT interval
Infrequently, a clinically significant increase in the QT interval was observed in patients receiving olanzapine, while there were no significant differences with placebo in the incidence of adverse cardiac events. However, as with the use of other antipsychotic drugs, it is recommended to exercise caution when prescribing Olanzapine Canon in combination with drugs that can prolong the QT interval, especially in elderly patients, with congenital prolongation of the QT interval, with congestive heart failure, myocardial hypertrophy, hypokalemia and hypomagnesemia.
Postural hypotension
Postural hypotension is uncommon in elderly patients. Just as when using other antipsychotics, when Olanzapine Canon is prescribed to patients over 65 years of age, it is recommended to monitor blood pressure.
Thromboembolism
The development of venous thromboembolism during olanzapine therapy is extremely rare. The presence of a cause-and-effect relationship between taking olanzapine and venous thromboembolism has not been established. However, given that patients with schizophrenia often have acquired risk factors for venous thromboembolism, it is necessary to conduct a cumulative assessment of all possible risk factors for the development of this complication, including immobilization of patients, and take the necessary preventive measures.
Liver dysfunction
In some cases, taking olanzapine, usually in the early stages of therapy, is accompanied by a transient, asymptomatic increase in hepatic transaminases (AST and ALT) in the blood serum. Rare cases of hepatitis have been reported. In very rare cases, hepatic cholestasis and other mixed liver damage have been observed. Particular caution is required when serum AST and/or ALT levels increase in patients with impaired liver function, with limited liver functional reserve, or in patients receiving treatment with potentially hepatotoxic drugs. If AST and/or ALT levels increase during treatment with olanzapine, careful monitoring of the patient is required and, if necessary, a reduction in the dose of Olanzapine Canon. If hepatitis, including hepatocellular, cholestatic or mixed, is detected, Olanzapine Canon should be discontinued.
Hyperglycemia and diabetes mellitus
There is a higher prevalence of diabetes mellitus in patients with schizophrenia. As with some other antipsychotic drugs, very rare cases of hyperglycemia, diabetes mellitus, exacerbation of pre-existing diabetes mellitus, ketoacidosis and diabetic coma have been reported. A cause-and-effect relationship between antipsychotic drugs and these conditions has not been established. Close clinical monitoring of patients with diabetes mellitus and patients with risk factors for developing diabetes mellitus is recommended.
For all groups of patients, regardless of body mass index, a clinically significant increase in body weight was observed. An increase of 7% or more from the mean after a short course of treatment (average duration of 47 days) was very common (22.2%), an increase of 15% or more was common (4.2%), and an increase of 25% or more was infrequent (0.8%).
In patients receiving longer treatment (at least 48 weeks), increases of 7%, 15%, and 25% were very common (64.4%, 31.7%, 12.3%, respectively).
Changes in lipid profile
In patients treated with olanzapine, undesirable changes in the lipid spectrum are observed. Monitoring of the lipid profile and clinical observation are recommended.
Epileptic seizures
Olanzapine Canon should be used with caution in patients with a history of epileptic seizures or those exposed to factors that lower the seizure threshold. In these patients, seizures are rare when treated with olanzapine.
Hematological changes
As with other antipsychotics, caution should be exercised when treating olanzapine in patients with a decreased number of leukocytes and/or neutrophils in the peripheral blood due to various reasons, with signs of depression or toxic impairment of bone marrow function under the influence of drugs in the anamnesis, with depression of function bone marrow due to concomitant disease, history of radiotherapy or chemotherapy, hypereosinophilia or myeloproliferative disease.
In clinical studies, the use of olanzapine in patients with a history of clozapine-dependent neutropenia or agranulocytosis was not accompanied by relapses of these disorders. The development of neutropenia has been reported primarily during concomitant therapy with olanzapine and valproic acid.
Anticholinergic activity
Olanzapine therapy is rarely associated with anticholinergic side effects. However, clinical experience with olanzapine in patients with concomitant diseases is limited, so caution is recommended when prescribing Olanzapine Canon to patients with clinically significant prostatic hypertrophy, paralytic ileus and similar conditions.
Dopaminergic antagonism
In vitro
Olanzapine exhibits dopamine receptor antagonism and, like other antipsychotics, may theoretically inhibit the effects of levodopa and dopamine agonists.
General activity in relation to the central nervous system
Given the primary CNS effects of olanzapine, caution should be exercised when using olanzapine in combination with other centrally acting drugs and alcohol.
Suicide
The risk of suicide attempts in patients with schizophrenia and bipolar disorder type 1 is determined by these diseases themselves. In this regard, during pharmacotherapy, careful monitoring of those patients whose risk of suicide is especially high is required. When prescribing Olanzapine Canon, one should strive to minimize the number of tablets taken by the patient in order to reduce the risk of overdose.
Cancellation of therapy
In case of abrupt withdrawal of olanzapine, sweating, insomnia, tremor, nausea and vomiting rarely (0.01-0.1%) develop. When discontinuing the drug, a gradual dose reduction is recommended.
Children and teenagers under 18 years of age
Olanzapine is not recommended for use in children and adolescents under 18 years of age due to the lack of sufficient data on efficacy and safety. In short-term studies that were conducted in adolescents 13-17 years of age, a greater increase in body weight and changes in lipid and prolactin concentrations were noted. than in similar studies in adults.