| Fluoxetine | |
| Fluoxetine | |
| Fluoxetine | |
| Chemical compound | |
| IUPAC | (RS)-N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine |
| Gross formula | C₁₇H₁₈F₃NO |
| Molar mass | 309.3 g/mol (345.8 for •HCl) |
| CAS | 54910-89-3 |
| PubChem | 3386 |
| DrugBank | APRD00530 |
| Classification | |
| Pharmacol. group | Antidepressants: Selective serotonin reuptake inhibitor |
| ATX | N06AB03 |
| Pharmacokinetics | |
| Bioavailable | ~72 % |
| Plasma protein binding | 94,5 % |
| Metabolism | Liver |
| Half-life | 1–3 days (fast phase); 4–6 days (slow phase); active metabolite norfluoxetine 4–16 days |
| Excretion | kidneys 80%, intestines 12–15% |
| Dosage forms | |
| capsules and tablets of 10 and 20 mg | |
| Method of administration | |
| inside | |
| Other names | |
| Prozac, Prodep, Profluzac, Fluval, Fluoxetine-Acree, Fluoxetine-Canon, Fluoxetine Hexal, Flunisan, Fluoxetine hydrochloride, Fluoxetine Lannacher, Apo-Fluoxetine, Fluxen | |
| Fluoxetine at Wikimedia Commons | |
Fluoxetine
(lat. Fluoxetine) is an antidepressant, one of the main representatives of the group of selective serotonin reuptake inhibitors. Its antidepressant effect is combined with a stimulating one. Improves mood, reduces tension, anxiety and fear, eliminates dysphoria. Does not cause orthostatic hypotension, sedation, and is not cardiotoxic. It has anorexigenic properties[1].
Fluoxetine is preferable for depression that occurs with motor retardation and hypersomnia; it may be poorly tolerated by patients with psychomotor agitation, anxiety and insomnia - it can aggravate these types of symptoms[2].
Fluoxetine was first registered in 1974 by scientists at Eli Lilly and Company. It was submitted to the US Food and Drug Administration in February 1977. Eli Lilly received final approval to market the drug in December 1987. Fluoxetine came out of patent protection in August 2001[3].
By 2011, despite the fact that many antidepressants appeared on the pharmacological market, fluoxetine occupied a leading position. Thus, in the United States in 2010, over 24.4 million prescriptions for fluoxetine were written,[4] making it the third most popular prescribed antidepressant after sertraline and citalopram[4]. In 2011, 6 million fluoxetine prescriptions were issued in the UK[5].
pharmachologic effect
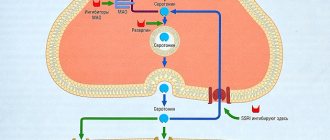
Fluoxetine is an antidepressant (bicyclic, 1-propylamine derivative) belonging to the selective or selective serotonin (5-HT) reuptake inhibitors at the synapses of CNS neurons. Has little effect on the reuptake of norepinephrine and dopamine. It has a weak effect on cholinergic, H1-histamine receptors and α-adrenergic receptors. Fluoxetine helps increase the concentration of serotonin in brain structures, which increases the duration of the stimulating effect of serotonin on the nervous system. By increasing serotonergic transmission, it inhibits neurotransmitter exchange through a negative feedback mechanism. With long-term use, it reduces the activity of 5-HT1 receptors. It also blocks the reuptake of serotonin in platelets.
Pharmacokinetics
When taken orally, it is well absorbed from the gastrointestinal tract (up to 95% of the dose taken), administration with food slightly inhibits the absorption of fluoxetine. The “first pass” effect through the liver is weakly expressed. Cmax in blood plasma is achieved after 6-8 hours. The bioavailability of fluoxetine after oral administration is more than 60%. The drug accumulates well in tissues and easily penetrates the BBB. Binding to blood plasma proteins is more than 90%. In the liver, enantiomers are demethylated with the participation of the cytochrome P450 isoenzyme CYP2D6 to norfluoxetine and other unidentified metabolites, and S
-norfluoxetine is equal in activity to
R
- and
S
-fluoxetine and superior to
R
-norfluoxetine. Excreted by the kidneys, the clearance of fluoxetine is 94-704 ml/min, norfluoxetine is 60-336 ml/min. Renal failure does not have a significant effect on the rate of elimination of fluoxetine. About 12% of the drug is excreted through the gastrointestinal tract. The half-life (T1/2) of fluoxetine is 1-3 days after a single dose and 4-6 days with long-term administration. T1/2 of norfluoxetine is 4-16 days in both cases, which causes significant accumulation of substances, slow achievement of their equilibrium level in plasma and prolonged presence in the body after withdrawal. In patients with liver cirrhosis, T1/2 of fluoxetine and its metabolites is prolonged. Excreted within 1 week mainly by the kidneys (80%): unchanged - 11.6%, in the form of fluoxetine glucuronide - 7.4%, norfluoxetine - 6.8%, norfluoxetine glucuronide - 8.2%, more than 20% - hippuric acid, 46% - other compounds; 15% is excreted by the intestines. If renal function is impaired, the elimination of fluoxetine and its metabolites slows down. It is not excreted during hemodialysis (due to the large volume of distribution and high degree of binding to plasma proteins). The drug is excreted in breast milk (up to 25% of the serum concentration).
The pharmacokinetics of fluoxetine explain the fact that its side effects may persist for a longer time than other SSRIs; the risk of developing serotonin syndrome due to drug interactions is also higher. Since the pharmacokinetics of fluoxetine is nonlinear, increasing its dose leads to a disproportionate increase in the level of the drug in the blood[6], accordingly to a disproportionately pronounced clinical effect and the same disproportionately pronounced side effects[7].
Cost of the drug
Fluoxetine-based medication is available only with a doctor's prescription. Its price varies depending on the manufacturer:
- Biocom company - from 28 to 38 rubles. per pack of 20 capsules, 10 mg of active ingredient;
- Russian company Alsi Pharma - from 37 to 56 rubles. for 20 pcs. 10 mg each;
- Ozone company - from 32 to 53 rubles. per pack of 20 capsules, 10 mg.
Packs of 20 capsules of 20 mg of active ingredient are slightly more expensive:
- Apotex Incorporated - from 110 rubles;
- Kanonpharma Production - from 108 to 130 rubles;
- Alsi Pharma - from 46 to 53 rubles;
- Biocom - from 36 to 44 rubles;
- Ozone - from 43 to 57 rubles.
The optimal duration of taking the drug is 2–3 months. Taking medication to lose weight will cost from 100 to 400 rubles. when using doses of 20 mg per day.
Application
Indications
Depression (regardless of the degree of depressive disorder - mild, moderate, severe), including in the structure of other mental disorders - schizophrenia, bipolar disorder, schizoaffective psychosis. Bulimia nervosa, alcoholism, obsessive-compulsive disorder.
Fluoxetine is especially effective with olanzapine and is produced in combination with it under the name Symbiax. for the treatment of bipolar depressive episodes and resistant depression[8][9].
There is evidence of the successful use of fluoxetine for chronic headaches[10].
Contraindications
Hypersensitivity to the drug, liver disease[11], chronic renal failure (creatinine clearance less than 10 ml/min.), bladder atony, glaucoma, prostate adenoma, decompensated epilepsy[12], history of convulsive reactions, diabetes mellitus[13] , manic states (both current[12] and in history[14]), poisoning with alcohol, psychotropic drugs and other drugs[15], pregnancy, breastfeeding[12], age less than 15 years[16].
With caution
Parkinson's disease, compensated renal and/or liver failure, cachexia.
Side effects
- From the nervous system:
dizziness, headache[15], drowsiness or insomnia, lethargy, increased fatigue, asthenia, bruxism, imbalance, depersonalization[17], irritability, dystonia[11], akathisia[15], tremor, agitation, increased anxiety[2], nervousness[12], aggressiveness[18], increased suicidal tendencies (typical of patients with depressive disorders), manic syndrome or hypomania, epileptic seizures[11], exacerbation of paranoid syndrome[16]. - From the digestive system:
loss of appetite, dry mouth or hypersalivation, nausea, diarrhea, dyspepsia, anorexia[11], vomiting[12]. - Allergic reactions
[15][16]: skin rash[12], urticaria. - Sexual dysfunction
is a common side effect of serotonin reuptake inhibitors. Specifically, it may include difficulty with sexual arousal, erectile dysfunction, loss of interest in sex, and anorgasmia (inability to achieve orgasm). Genital insensitivity[19], lost or decreased response to sexual stimuli, and anhedonia during ejaculation are also possible. Although sexual side effects are usually reversible, they may last for months, years, or even permanently after stopping the drug[20]; This situation is called post-SSRI sexual dysfunction (English)Russian.. - Other:
hyperprolactinemia[21], hyponatremia[12], impaired visual acuity[15], gynecological bleeding, alopecia, dysuria[17], dysphonia, pharyngitis, feeling of heat[12], serotonin syndrome (in high doses), arthropathy (rarely )[16], increased sweating, weight loss, systemic disorders of the lungs, kidneys or liver, vasculitis. - When using fluoxetine (extremely rarely), there have been cases of a deadly side effect - neuroleptic malignant syndrome, which occurs most often when taking antipsychotics [7].
- Taking fluoxetine in the first trimester of pregnancy increases the risk of cardiac development disorders in the fetus[22].
Withdrawal syndrome
As with some other SSRIs and SSRIs (most often when stopping paroxetine), withdrawal symptoms may occur within 1-7 days after stopping the drug or reducing the dosage. The most common symptom during drug withdrawal is dizziness. Nausea or vomiting, fatigue, headache, unsteadiness in gait, and insomnia may also occur; The least common symptoms that develop are anxiety, diarrhea, irritability, tremor, paresthesia, and visual disturbances. To diagnose withdrawal syndrome, two or more of the listed symptoms are sufficient[23]. In these cases, a more gradual dose reduction is necessary; As a rule, withdrawal symptoms disappear within 72 hours.
Signs of overdose
If you do not follow the dosage prescribed by your doctor, an overdose with the following symptoms is possible:
- nausea and vomiting, stomach pain;
- limb spasms;
- feeling of fear, anxiety;
- epilepsy attack.
In case of overdose of the drug, gastric lavage and sorbents are prescribed. If seizures or epilepsy occur, the doctor will prescribe appropriate therapy.
special instructions
Use fluoxetine with caution in patients with acute cardiovascular disease[12].
When treating patients with underweight, anorexigenic effects should be taken into account (progressive weight loss is possible). In patients with diabetes mellitus, the use of fluoxetine increases the risk of developing hypoglycemia and hyperglycemia when it is discontinued. In this regard, the dose of insulin and/or any other hypoglycemic drugs used orally should be adjusted.
Until significant improvement in treatment occurs, patients should be under medical supervision.
During treatment with fluoxetine, you should refrain from drinking alcohol. Fluoxetine is prescribed with caution for activities that require a high concentration of attention and speed of reactions [13].
In old age, treatment with fluoxetine should begin with 1/2 dose.
Withdrawal syndrome
If a person takes Fluoxetine uncontrollably, he may become addicted. In this case, a completely logical question arises - how to get off Fluoxetine? There is only one way out - you need to abandon it immediately. But in this case, there is a high probability of developing withdrawal syndrome.
By the way, withdrawal syndrome can appear without addiction if the therapy was long-term and the drug was discontinued abruptly. Statistics show that in more than half of the cases (more precisely, 60%), side effects begin when treatment is stopped. Most often they manifest themselves as dizziness. But it is also possible:
- sensory disturbances;
- interruptions in sleep (from deep sleep to complete insomnia);
- asthenia;
- anxiety;
- excitation;
- disorders of the gastrointestinal tract and so on.
As a rule, these conditions are not dangerous, and the symptoms gradually go away on their own within a couple of weeks. But sometimes the symptoms increase and last for quite a long time - several months. This condition requires specialist intervention.
To avoid the syndrome, it is carried out gradually and completely abandoned in 1-2 weeks.
Drug interactions
Enhances the effects of diazepam, ethanol and hypoglycemic drugs, tricyclic antidepressants, trazodone, alprazolam, triazolam, beta-blockers, carbamazepine, sodium valproate[24], phenytoin[11], barbiturates[25]. Reduces the anti-anxiety effect of buspirone (English) Russian. It is not recommended to combine with trazodone, bupropion, alprazolam, buspirone, lithium[16]. Changes the concentration of lithium in the blood[12], resulting in an increased risk of neurotoxicity[26].
When fluoxetine is combined with tricyclic antidepressants, the effect of cardiotoxicity increases sharply, as the concentration of tricyclic antidepressants in the blood increases [26]. It is not recommended to combine with imipramine, desipramine, nortriptyline[16]. Studies have shown that the steady-state concentrations of imipramine and desipramine in the blood after the addition of fluoxetine increase by 2-10 times. Even after fluoxetine is discontinued, this effect may take up to 3 weeks to occur because fluoxetine has a long half-life[11].
The simultaneous use of fluoxetine and typical antipsychotics leads to an increased risk of developing extrapyramidal symptoms[26] and to its intensification. It is not recommended to combine fluoxetine with haloperidol[16].
Fluoxetine may increase the severity of psychomotor impairment if it is used in conjunction with benzodiazepine tranquilizers[26].
Fluoxetine causes an increase in the level of the main metabolite of bupropion - hydrox-bupropion, which can lead to clinical manifestations of the toxic effects of this metabolite: catatonia, confusion and agitation [7].
Macrolide antibiotics (erythromycin, clarithromycin, etc.) increase the concentration of fluoxetine in the blood with the possible development of toxic phenomena [24]; Clarithromycin in combination with fluoxetine can cause psychosis[26]. Tryptophan enhances the serotonergic properties of fluoxetine, therefore increased consumption of tryptophan, including through food, can cause increased agitation, motor restlessness, and gastrointestinal disorders.
Fluoxetine in combination with statins can cause manifestations of myositis[26].
Taking fluoxetine with mirtazapine, according to a 2009 study of 105 patients, doubled the number of patients achieving long-term, sustained remission compared with therapy with one drug[27]. Similar combinations are used for resistant (treatment-resistant) depression. It is also possible to use fluoxetine together with bupropion for these purposes, since they do not belong to the same group of antidepressants.
Antidepressants - MAO inhibitors should not be used in conjunction with fluoxetine due to the possible development of serotonin syndrome [28]. Treatment with fluoxetine can be started at least 14 days after discontinuation of irreversible MAO inhibitors[13]. Between the end of treatment with fluoxetine and the start of therapy with irreversible MAO inhibitors, a gap of at least 5 weeks should be maintained, and in elderly patients - at least 8 weeks. A gap of at least 2 weeks is required between stopping fluoxetine and starting other SSRIs[29].
Serotonin syndrome can also occur when SSRI antidepressants are combined with clomipramine, amitriptyline, trazodone, buspirone, levodopa[30], herbal antidepressant drugs containing St. John's wort[31], with 5-hydroxytryptophan, S-adenosylmethionine (SAM, heptral)[32] and tryptophan[33], dextromethorphan, tramadol[29] and other opioid analgesics[34], carbamazepine, lithium preparations[29], metoclopramide[35].
When fluoxetine was used together with calcium channel blockers (verapamil, nifedipine), headaches, swelling, and nausea were observed [36].
Medicines that have a depressant effect on the central nervous system, in combination with fluoxetine, increase the risk of side effects and increased depressant effects on the central nervous system. With the simultaneous use of drugs with a high degree of protein binding, especially with anticoagulants and digitoxin, it is possible to increase the plasma concentration of free (unbound) drugs and increase the risk of adverse effects.
Fluoxetine analogs
There are two common brands in Russia: Fluoxetine-Lannacher and Canon.
There are many drugs based on the active substance “fluoxetine”:
- Prozac
- Profluzak
- Prodep
- Fluval
- Flunisan
- Fluxen and others.
Some compare Fluoxetine with Amitriptyline, although the latter drug is a tricyclic antidepressant, which has a sedative effect.
Analogs belonging to the SSRI group of antidepressants are also used:
- Alaprolate
- Bupropion
- Vilazodone
- Venlafaxine
- Duloxetine
- Dapoxetine
- Desvenlafaxine
- Zimelidin
- Zoloft (Sertraline)
- Indalpin
- Levomilnacipran
- Milnacipra
- Paxil (Paroxetine)
- Citalopram
- Fevarin (Fluvoxamine)
- Etoperidone and others.
Criticism
Cases of suicide and other types of aggressive behavior while taking fluoxetine, as well as publications in the media and lawsuits against the pharmaceutical company Eli Lilly and Company on this issue, have become widely known in the United States. A total of 70 lawsuits have been filed against Eli Lilly. In all cases, it was stated that the patients did not experience suicidal tendencies before taking this drug.[37] Internal company documents show that Eli Lilly for a long time hid information about suicides due to taking Prozac in clinical trials and explained them as overdoses or manifestations of depression[38]. As it turned out, the company had known since 1978 that fluoxetine could cause a strange agitated state in some patients, causing them to commit suicide or murder. Specifically, Eli Lilly excluded 76 of 97 suicides in Prozac users from a post-marketing observational study submitted to the FDA.[39]
By 1999, the FDA had received reports of more than 2,000 suicides associated with fluoxetine, and a quarter of the reports clearly indicated the development of agitation and akathisia in patients, which also carry an increased risk of suicide. In 2006, the EMA (European Medicines Agency) announced that parents and doctors should closely monitor children and adolescents treated with fluoxetine, especially for suicidal tendencies[39].
By 2000, the amount of compensation Eli Lilly was forced to pay in connection with Prozac reached $50 million. Lawsuits have also been filed in connection with the side effects of Symbiax (English)Russian. - a combination drug that includes olanzapine and fluoxetine. According to some patients who took Symbiax during pregnancy, the drug can cause birth defects such as cleft lip and palate, spina bifida, anencephaly, and clubfoot[40].
The book by the famous American psychologist Irving Kirsch, “The Emperor's New Drugs: Busting the Antidepressant Myth,” describes the results of a review of 42 clinical trials of 6 antidepressants, including 4 SSRI antidepressants - fluoxetine, paroxetine, sertraline and citalopram. Data from some of these studies have not been published before, and their results were kept silent. After analyzing the studies, Kirsch noted that the difference between drugs and placebo averaged only 1.8 points on the Hamilton scale - a difference, although statistically significant, was clinically meaningless[41]. According to another study by Kirsch et al (a meta-analysis of 35 clinical trials of 4 antidepressants, including fluoxetine), the difference between antidepressants and placebo reached clinical significance only in very severe depression[42]. The results of Kirsch's research caused widespread resonance and were discussed both in scientific journals and in the popular media[43].
Peter Goetsche (English) Russian, one of the founders of Cochrane, professor of clinical trial design and analysis at the University of Copenhagen, author of more than 70 articles in leading medical journals, calls fluoxetine a “terrible drug” and cites evidence that senior management of Eli Lilly in the late 1980s, they initially wanted to delay its release, and only the fact that the company was then in a deep financial crisis led to the release of the drug on the market. According to Goetsche, the German drug regulator gave the following conclusion based on the results of its evaluation of fluoxetine: “After comparing the benefits and risks, we came to the conclusion that the drug is completely unsuitable for the treatment of depression.” In order to get the drug approved in Sweden (which would have a positive impact on the FDA's decision on Prozac), doctors were first invited to a Caribbean resort for a week and then paid a bribe of $20,000. At the same time, references to lethal side effects disappeared from the clinical trial data provided in the registration application, and the original wording “Five subjects had hallucinations and attempted suicide, which four succeeded in doing” was changed to “The other five subjects different effects were observed”[39].
In addition, Eli Lilly bribed members of an FDA advisory panel that was convened in 1991 to review data on fluoxetine. The advisory group concluded that fluoxetine is safe, despite concerns raised by safety expert David Graham and several others.[39]
As Peter Goetsche notes, Eli Lilly illegally promoted fluoxetine for some unapproved indications, such as shyness, eating disorders, and low self-esteem. Bias in industry-sponsored trials of fluoxetine is very large: in head-to-head trials in which Prozac was the main subject of study, significantly more patients benefited from it than in trials in which Prozac was the comparator (used for comparison)[39].
Which manufacturer is better?
Fluoxetine is produced by both foreign and domestic manufacturers. Among the foreign ones, the most famous are:
- Patheon France. The trade name of the drug is Prozac. The product was developed in Switzerland, but is currently produced in France. Among the advantages are many tests conducted in clinical settings, which confirmed that the drug is effective and, most importantly, safe. The disadvantage is the high cost.
- "G. L. Pharma.” The manufacturing company is located in Austria. Price category – average.
- "Apotex". Large Canadian pharmaceutical company. Among foreign drugs it has the most reasonable price.
Many domestic companies are also involved in the production of Fluoxetine. Each of them has all the necessary quality certificates, so the products meet European quality standards.
It’s hard to say which manufacturer is better. It is not entirely correct to compare them. The fact is that all drugs (regardless of whether it is Prozac, Fluoxetine or another) have the same action, properties and indications for use. The difference, as a rule, lies only in the speed of onset of the effect, which depends on the methods of purification of raw materials - each manufacturer has their own.
Many specialists who treat obsessive and depressive disorders prefer the original drug, Prozac, arguing that this drug has more clinical trials and proven effects. But there are drugs from other manufacturers that are no less effective than Prozac, at a lower cost. They are in great demand.
Notes
- Kharkevich D. A.
Pharmacology. — 10th ed. - M.: GEOTAR-Media, 2010. - P. 642. - 908 p. — ISBN 978-5-9704-0850-6. - ↑ 1 2 Drobizhev M. Yu., Mukhin A. A.
Selective serotonin reuptake inhibitors: options for choice (comments on the works of Thase et al.) // Psychiatry and psychopharmacotherapy. - 2004. - T. 6, No. 1. - 'Generic Prozac' expected to be cleared for sale. CNN (1 August 2001). Retrieved December 27, 2012.
- ↑ 1 2
Top 200 Generic Drugs by Units in 2010 (English) (PDF).
Verispan
. Drug Topics. Archived from the original on December 15, 2012. - Patrisha Macnair.
BBC - Health: Prozac (English). BBC (September 2012). — “In 2011 over 43 million prescriptions for antidepressants were handed out in the UK and about 14 per cent (or nearly 6 million prescriptions) of these were for a drug called fluoxetine, better known as Prozac.” Archived from the original on February 9, 2013. - Ushkalova E. A., Ushkalova A. V.
Pharmacotherapy of depression in cardiac patients // Difficult patient. - 2006. - No. 1. Archived on May 22, 2013. - ↑ 1 2 3 Janiczak F. J., Davis J. M., Preskorn S. H., Ide F. J. Jr.
Principles and practice of psychopharmacotherapy. - 3rd. - M., 1999. - 728 p. — ISBN 966-521-031-9. - (2007-02) "A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder." The Journal of Clinical Psychiatry 68
(2): 224–36. DOI:10.4088/jcp.v68n0207. PMID 17335320. - Grohol, J.
FDA Approves Symbyax for Treatment of Resistant Depression.
Psych Central Blog
. - Voznesenskaya T.G.
Treatment of chronic headaches with Prozac // Klin. Pharmacol. and therapy. - 1999. - No. 1. - P. 34-36. - ↑ 123456
Pharmacotherapy in neurology and psychiatry: [Trans. from English] / Ed. S. D. Enna and J. T. Coyle. - M.: Medical Information Agency LLC, 2007. - 800 p.: ill. With. — 4000 copies. — ISBN 5-89481-501-0. - ↑ 1 2 3 4 5 6 7 8 9 10 Gubsky Yu. I., Shapovalova V. A., Kutko I. I., Shapovalov V. V.
Medicines in psychopharmacology. - Kyiv - Kharkov: Health - Torsing, 1997. - 288 p. — 20,000 copies. — ISBN 5-311-00922-5, 966-7300-04-8. - ↑ 1 2 3 Mashkovsky M.D.
Medicines. — 16th ed., revised, corrected. and additional - Moscow: New Wave, 2012. - 1216 p. — 5000 copies. — ISBN 978-5-7864-0218-7. - Ushkalova A. V., Kostyukova E. G., Mosolov S. N.
Problems of diagnosis and treatment of bipolar depression: from evidence-based scientific research to clinical recommendations // Biological methods of treatment of mental disorders (evidence-based medicine - clinical practice) / Ed. S.N. Mosolova. - M.: Publishing house "Socio-political thought", 2012. - P. 529-553. — 1080 s. — 1000 copies. — ISBN 978-5-91579-075-8. - ↑ 12345
Puzhinsky, 1997. - ↑ 1 2 3 4 5 6 7 Podkorytov V. S., Chaika Yu. Yu.
Depression. Modern therapy. - Kharkov: Tornado, 2003. - 352 p. — ISBN 966-635-495-0. - ↑ 12
Information letter from CEBLS on the safety of fluoxetine. Retrieved December 24, 2014. - Ener RA, Meglathery SB, Van Decker WA, Gallagher RM
Serotonin syndrome and other serotonergic disorders. (English) // Pain medicine (Malden, Mass.). - 2003. - Vol. 4, no. 1. - P. 63-74. - PMID 12873279. [correct] - (2006) "Genital Anaesthesia Persisting Six Years after Sertraline Discontinuation." Journal of Sex & Marital Therapy 32
(4): 327–30. DOI:10.1080/00926230600666410. PMID 16709553. - (2007) “Persistent Sexual Dysfunction after Discontinuation of Selective Serotonin Reuptake Inhibitors.” Journal of Sexual Medicine 5
(1): 227–33. DOI:10.1111/j.1743-6109.2007.00630.x. PMID 18173768. - Torre D, Falorni A.
Pharmacological causes of hyperprolactinemia // Ther Clin Risk Manag. - Oct 2007. - T. 3, No. 5. - P. 929-951. - Paroxetine (Paxil) and fluoxetine (Prozac) can cause fetal development disorders // Moscow Regional Psychiatric Newspaper. — January — February 2009 — No. 1 (45). (unavailable link)
- Black K, Shea C, Dursun S, Kutcher S (May 2000). "Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria." J Psychiatry Neurosci 25
(3):255–61. PMID 10863885. - ↑ 1 2 Malin D.I.
Drug interactions of psychotropic drugs (Part II) // Psychiatry and psychopharmacotherapy. - 2000. - Sirolo D., Shader R., Grinblatt D.
Chapter 16. Drug interactions of psychotropic drugs // Psychiatry / ed. Sheider R. (translated from English by M. V. Pashchenkova with the participation of D. Yu. Veltishchev; edited by N. N. Alipov). - Practice, 1998. - ISBN 5-89816-003-5. - ↑ 1 2 3 4 5 6 Minutko V.L.
Depression. - Moscow: GEOTAR-Media, 2006. - 320 p. — 2000 copies. — ISBN 5-9704-0205-2. - Combination of Antidepressant Medications From Treatment Initiation for Major Depressive Disorder: A Double-Blind Randomized Study (inaccessible link)
- Mosolov S. N., Kostyukova E. G., Serditov O. V.
Serotonin syndrome in the treatment of depression // International Journal of Medical Practice. - MediaSphere, 2000. - No. 8. Archived on October 4, 2013. - ↑ 1 2 3 Mosolov S.N., Kostyukova E.G., Serditov O.V.
Serotonin syndrome in the treatment of depression // International Journal of Medical Practice. - MediaSphere, 2000. - No. 8. Archived on October 4, 2013. - Schlienger RG, Shear NH.
Serotonin syndrome // British Journal of Psychiatry. - 1996. - T. 169 (suppl.31). — P. 15-20. Translation: Serotonin syndrome // Review of modern psychiatry. - 1998. - Issue. 1. - Antidepressant therapy and other treatments for depressive disorders: Report of the CINP Working Group based on a review of evidence / Editors T. Bagai, H. Grunze, N. Sartorius. The translation into Russian was prepared at the Moscow Research Institute of Psychiatry of the Russian Federal Healthcare Service under the editorship of V.N. Krasnova. - Moscow, 2008. - 216 p. Archived from the original on March 4, 2020.
- Drug Interactions: SSRIs. iHerb.Com. Archived March 15, 2012.
- Arana J., Rosenbaum J.
Pharmacotherapy of mental disorders. Per. from English - M.: BINOM Publishing House, 2004. - 416 p. — ISBN 5-9518-0098-6. - Bykov Yu. V., Bekker R. A., Reznikov M. K.
Resistant depression. Practical guide. - Kyiv: Medkniga, 2013. - 400 p. — ISBN 978-966-1597-14-2. - Fisher AA, Davis MW (January 2002). "Serotonin Syndrome Caused by Selective Serotonin Reuptake-Inhibitors-Metoclopramide Interaction." The Annals of Pharmacotherapy 36
(1): 67–71. PMID 11816261. - Mosolov S. N.
Basics of psychopharmacotherapy. - Moscow: Vostok, 1996. - 288 p. - Chetley E.
Problem Drugs = Problem Drugs. — per. from English - Riga, Latvia: Landmark, 1998. - 352 p. — ISBN 9984-9066-2-0. - Leo J, Lacasse JR (February 2008). "The Media and the Chemical Imbalance Theory of Depression". Society 45
(1): 35–45. DOI:10.1007/s12115-007-9047-3. - ↑ 1 2 3 4 5 Götsche P.
Deadly drugs and organized crime: How Big Pharma corrupted healthcare / [Trans. from English L. E. Ziganshina]. - Moscow: Publishing House "E", 2020. - 464 p. — (Evidence-based medicine). — 3000 copies. — ISBN 978-5-699-83580-5. - Eli Lilly & Co. — Drug Manufacturer's History, Products, Lawsuits (English). Drugwatch.com (November 2, 2015). Retrieved May 2, 2016.
- Angell M.[en].
The Epidemic of Mental Illness: Why?. The New York Review of Books (January 23, 2011). Archived from the original on September 21, 2012. - Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (February 2008). "Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration." PLoS Medicine 5
(2):e45. DOI:10.1371/journal.pmed.0050045. PMID 18303940. - Horder J, Matthews P, Waldmann R (June 2010). "Placebo, Prozac and PLoS: significant lessons for psychopharmacology." Journal of Psychopharmacology 25
(10): 1277–88. DOI:10.1177/0269881110372544. PMID 20571143.