In our difficult times, the number of individuals suffering from depressive disorders increases every year. In this state of affairs, modern antidepressant drugs become especially relevant. One of the best such medications is Remeron, instructions for use for which are given in this article. Readers interested in this topic will be interested in learning about the composition of the drug, its properties, method of use and, of course, how doctors respond to this remedy.
Composition of the drug and release form
The medicine is available in reddish-brown, biconvex tablets, each of which contains 15, 30 or 45 grams of the active substance, mirtazapine. On one side of the tablets there is a marking Organon - this is the name of the Dutch manufacturer of this drug, on the other side you can see the code TZ/5. All of the above relates exclusively to the antidepressant marketed under the brand name Remeron. Drug analogues, a list of which can be read in our article, may have a composition almost identical to Remeron, but their name and appearance will be different.
Dosage and application regimen
The medicine is prescribed at an initial dose of 15 milligrams per day. Gradually, the dose can be increased to 45 milligrams.
It is recommended to take the medicine in the evening, preferably before going to bed, once a day. It is not advisable to chew the tablets. It is also possible to take the medicine twice a day if necessary. In this case, the entire daily dose is divided into two parts. The minimum dose is taken in the morning, and the maximum dose is drunk before bed.
The duration of treatment is from four months to six months, until clinical signs begin to disappear. If no improvement is observed within two months, then therapy is stopped.
Withdrawal syndrome is possible in case of abrupt refusal of the medication.
The dosage and treatment regimen are determined by a qualified specialist, taking into account the severity of the pathology, as well as the individual characteristics of the body.
New generation antidepressant
Why do psychiatrists today, if patients come to them about depression, panic attacks, etc., often prescribe Remeron? The instructions for use describe in sufficient detail the mechanism of its action, but, unfortunately, it does not indicate that this is a new generation antidepressant, during the development of which many factors were taken into account to make the drug as effective as possible and at the same time reduce side effects.
In a scientific article devoted to mirtazapine, the main active ingredient included in Remeron, a psychotherapist and employee of the Institute of Gerontology in Kiev, S. G. Burchinsky writes that this drug is distinguished by the following important characteristics:
- the clinical effect after starting to take the medicine occurs very quickly (almost in the first week of use);
- pronounced anxiolytic and thymoanaleptic effect;
- sedative effect and normalization of sleep.
Reviews about Remeron
Judging by reviews from doctors, the drug is most often prescribed for panic attacks and vegetative-vascular dystonia . The medicine is well tolerated, brings patients back to life, improves sleep and appetite. Sometimes, to eliminate side effects from taking it, additional medications are prescribed. It should be remembered that Remeron is a fairly serious medicine and should not be taken without the consultation and recommendations of the attending physician.
Reviews about Remeron on the forums:
“The pills are good for relieving depression. Depression is endogenous, it is useless to look for reasons. I can only save myself with medicine. Disadvantage: increased appetite”;
“My attacks - breathing problems, migraines, dizziness - Remeron relieved me perfectly. But it also has its own “side effects”: it’s impossible to sit still and it’s impossible to stop eating! I was constantly running around and couldn’t sleep. And she began to gain weight by leaps and bounds, even while limiting herself in food”;
“Switched to it from another medicine. The difference is amazing! It’s nice to feel like a real person again.”
Effect of the drug on sleep structure
"Remeron" is able to effectively influence depressive symptoms that are difficult to correct with other antidepressants (cyclothymic disorders, psychomotor retardation, anhedonia). Doctors note that mirtazapine is extremely effective both in the treatment of acute depressive episodes and as part of maintenance therapy. Its influence on the normalization of sleep is especially emphasized.
It is known that in depressive disorders, sleep disturbance is one of the typical symptoms of this pathology and is observed in almost 90% of patients. These disorders consist of disturbances during sleep onset and awakening, as well as disorganization of the overall sleep structure. Addressing this problem is a very important component in helping those suffering from depression. For the majority of such people who visit a medical facility, there is a clear relationship between the severity of insomnia and the severity of affective symptoms.
At the same time, sleep disturbances can seriously complicate the course of the pathological process, as well as precede and accompany the disease. They may subsequently increase the risk of relapse. Based on the above, we can conclude that therapeutic measures taken to normalize sleep in patients with depression should be not so much symptomatic as pathogenetic. Remeron, created on the basis of mirtazapine, is one of the rare antidepressants that can successfully correct insomnia disorders in patients with depression.
Mode of application
The tablets should be taken orally, with liquid if necessary, and swallowed without chewing. Adults: The effective daily dose is usually between 15 and 45 mg; the initial dose is 15 or 30 mg. Elderly: The recommended dose is the same as for adults. In elderly patients, in order to achieve a satisfactory and safe response to treatment, increasing the dose should be done under the direct supervision of a physician. In patients with renal or hepatic impairment, the clearance of mirtazapine may be reduced. This should be taken into account when prescribing Remeron to this category of patients. Remeron can be taken once a day, preferably at the same time, before bedtime. Remeron can also be prescribed to be taken 2 times a day, dividing the daily dose in half (1 time in the morning and 1 time at night, the higher dose should be taken at night). Treatment should, if possible, be continued for 4-6 months until the patient's symptoms disappear completely. After this, treatment can be gradually withdrawn. Mirtazapine usually begins to have its effect after 1-2 weeks of treatment. Treatment with an adequate dose should lead to a positive response within 2-4 weeks. If the response to treatment is insufficient, the dose can be increased to the maximum dose (up to 45 mg). If there is no response to treatment, treatment should be discontinued after another 2-4 weeks.
Application in geriatric practice
According to the observations of doctors, Remeron is very well tolerated by elderly and senile people, despite the fact that it has a very effective and beneficial effect on this category of patients. This is an undoubted advantage of the drug, since many of the symptoms that accompany depression (anhedonia, anxiety, sleep disturbances, etc.) especially often plague people in old age. Considering the fact that in this age group the use of TADs (tricyclic antidepressants) is not recommended, in gerontopsychiatry Remeron is used in the treatment of literally all forms of depressive pathologies.
pharmachologic effect
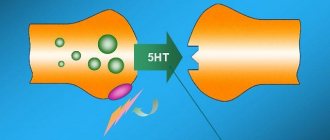
Remeron (mirtazapine) is a tetracyclic antidepressant with a predominantly sedative effect. The drug is most effective for depressive conditions with the presence in the clinical picture of symptoms such as an inability to experience pleasure and joy, loss of interest (anhedonia), psychomotor retardation, sleep disturbances (especially in the form of early awakenings) and weight loss, as well as other symptoms: suicidal thoughts and daily mood fluctuations. The antidepressant effect of Remeron usually occurs after 1-2 weeks of treatment. Mirtazapine is an antagonist of presynaptic β2-adrenergic receptors in the central nervous system and enhances central noradrenergic and serotonergic transmission of nerve impulses. In this case, the enhancement of serotonergic transmission is realized only through serotonin 5-HT1 receptors, since mirtazapine blocks serotonin 5-HT2 and 5-HT3 receptors. Both enantiomers of mirtazapine are believed to have antidepressant activity, the S(+) enantiomer by blocking β2-adrenergic and serotonin 5-HT2 receptors, and the R(-) enantiomer by blocking serotonin 5-HT3 receptors. The sedative properties of mirtazapine are due to its antagonistic activity towards histamine H1 receptors. Mirtazapine is generally well tolerated. In therapeutic doses, it has virtually no anticholinergic effect and has virtually no effect on the cardiovascular system.
Correction of sexual disorders
For sexual disorders such as anorgasmia, accelerated ejaculation, decreased libido, etc., which quite often accompany clinical manifestations of depression on the one hand, and on the other hand, often arise as complications during treatment with SSRI drugs (selective serotonin reuptake inhibitors), “ Remeron" and its analogues serve as an excellent help for doctors. Mirtazapine-based drugs are excellent for correcting a wide variety of sexual dysfunctions in patients with depression. At the same time, they not only increase the effectiveness of treatment, but also act as prophylactics against the development of sexual disorders.
Interaction
Pharmacokinetic interaction - mirtazapine is extensively metabolized with the participation of the CYP2D6 and CYP3A4 isoenzymes, and to a lesser extent with the participation of the CYP1A2 isoenzyme. An interaction study in healthy volunteers showed that paroxetine, an inhibitor of the CYP2D6 isoenzyme, has no effect on the pharmacokinetics of mirtazapine at steady state. Administration in combination with a strong CYP3A4 inhibitor, ketoconazole, increased the maximum plasma concentration and AUC of mirtazapine by approximately 40% and 50%, respectively. Caution should be exercised when mirtazapine is used in combination with strong CYP3A4 inhibitors, HIV protease inhibitors, azole antifungals, erythromycin, or nefazodone. - carbamazepine and phenytoin, inducers of the CYP3A4 isoenzyme, increased the clearance of mirtazapine by approximately two times, which led to a 45-60% decrease in plasma concentrations of mirtazapine. When adding carbamazepine or another inducer of hepatic metabolism (eg, rifampicin) to mirtazapine therapy, the dose of mirtazapine should be increased if necessary. If treatment with this drug is stopped, the dose of mirtazapine may need to be reduced. - when used in combination with cimetidine, the bioavailability of mirtazapine can increase by more than 50%. The dose of mirtazapine should be reduced if necessary when starting treatment in combination with cimetidine or increased when treatment with cimetidine is discontinued. - In in vivo interaction studies, mirtazapine had no effect on the pharmacokinetics of risperidone or paroxetine (CYP2D6 substrate), carbamazepine (CYP3A4 substrate), amitriptyline, cimetidine or phenytoin. - No important clinical effects or changes in pharmacokinetics were observed in humans when treated with mirtazapine in combination with lithium. Pharmacodynamic interactions: Mirtazapine should not be used in combination with MAO inhibitors or within two weeks after stopping treatment with a MAO inhibitor. - Mirtazapine may enhance the sedative properties of benzodiazepines and other sedatives. Caution should be exercised when prescribing these drugs with mirtazapine. - Mirtazapine may enhance the depressive effect of alcohol on the central nervous system. Therefore, patients should be warned to avoid drinking alcohol. - in the case of using other serotonergic drugs (for example, selective serotonin reuptake inhibitors and venlafaxine) in combination with mirtazapine, there is a risk of interaction, which can lead to the development of serotonin syndrome. Based on post-registration experience with the drug, it turned out that serotonin syndrome occurs very rarely in patients receiving treatment with mirtazapne in combination with selective serotonin reuptake inhibitors or venlafaxine. If such a combination is considered therapeutically necessary, the dose should be adjusted cautiously and closely monitored for signs of the onset of sustained serotonergic overstimulation. Mirtazapine 30 mg once daily caused a small but statistically significant increase in INR in subjects treated with warfarin. A more pronounced effect with a higher dose of mirtazapine cannot be excluded. It is recommended to monitor MHO in case of treatment with warfarin in combination with mirtazapine.
"Remeron": instructions for use
The package insert that comes with the medicine states that you should take the medicine once a day just before bed. The daily standard dose can vary between 15-45 mg, and no special adjustment of the amount of the drug is required for the elderly. It is very important not to overdose on Remeron. Reviews from patients confirm what is written in the instructions: an overdose does not cause an improvement in the condition, but lethargy and drowsiness. During treatment, it is important to refrain from drinking alcoholic beverages.
Increased sedative action can be caused by simultaneous use of the drug with benzodiazepines. It is not recommended to take Remeron tablets - 30, 15 and 45 grams together with MAO inhibitors, or after stopping the latter for fourteen days. The instructions recommend continuing treatment with Remeron even after the disappearance of painful symptoms; This must be done for six months. Discontinuation of the drug should be carried out gradually. Important: this medicine is sold in pharmacies only with a doctor's prescription!
Pharmacological properties of the drug Remeron
Pharmacodynamics. Mirtazapine is an antagonist of presynaptic α2 receptors in the central nervous system and enhances central noradrenergic and serotonergic transmission of nerve impulses. Enhancement of serotonergic transmission occurs exclusively through 5-HT1 receptors, since mirtazapine blocks 5-HT2 and 5-HT3 receptors. Both spatial enantiomers of mirtazapine exhibit antidepressant activity, with the S(+) enantiomer blocking α2 and 5-HT2 receptors, and the R(-) enantiomer blocking 5-HT3 receptors. In addition, mirtazapine blocks H1 receptors, which determines its sedative properties. In therapeutic doses, mirtazapine exhibits virtually no anticholinergic activity and does not affect the cardiovascular system. Pharmacokinetics. After oral administration, mirtazapine is rapidly absorbed. Bioavailability is approximately 50%. The maximum plasma concentration is observed after approximately 2 hours. Approximately 85% of mirtazapine is bound to plasma proteins. The half-life averages 20–40 hours; in some cases - up to 65 hours. Usually, in young people, the half-life is shorter than in older people. The long half-life allows the drug to be taken once a day. The equilibrium concentration of the active substance in the blood plasma is achieved after 3–4 days; thereafter it does not change. In the range of recommended doses, the pharmacokinetic parameters of mirtazapine have a linear dependence on the dose. Mirtazapine is actively metabolized and excreted from the body in urine and feces over several days. The main routes of its metabolism in the body are demethylation and oxidation with further conjugation. Demethylmirtazapine is also pharmacologically active. The clearance of mirtazapine may be reduced in patients with renal or hepatic impairment.
Contraindications
"Remeron" is a drug that cannot be used by people who are hypersensitive to its main active component - the substance mirtazapine, as well as to any of the auxiliary components of the drug. The following contraindications are pregnancy, lactation, renal or liver failure. There are also relative contraindications:
- prostatic hypertrophy;
- diabetes;
- angle-closure glaucoma;
- atrial fibrillation;
- arterial hypotension;
- angina pectoris;
- organic lesions of the brain (brain);
- tendency to abuse psychoactive drugs.
During pregnancy and lactation
Experience with the use of the drug in pregnant women is limited. Animal studies have not shown any effects on the child's health. The question of prescribing the drug to pregnant women should be decided by the attending physician.
the mother took antidepressants during pregnancy , a postnatal examination of the newborn should be performed after birth to exclude the development of withdrawal syndrome.
The active ingredient of the drug passes into breast milk in small quantities. The decision to stop breastfeeding should be made after consultation with a specialist.
"Remeron": reviews from doctors
Experts highly value the pronounced therapeutic properties of this new generation antidepressant. Before the drug became part of everyday medical practice, the effects of mirtazapine were studied very carefully. Thus, there is an article by M. A. Morozova, an employee of the Moscow Scientific Center for Mental Health of the Russian Academy of Medical Sciences, where she talks about the results of such studies.
The trials involved 63 patients with moderate to severe depression, with various types of affective disorders identified; 12 psychiatric hospitals in different Russian cities were involved. As part of the experiment, doctors were instructed to use only mirtazapine to treat all patients hospitalized with depression. The total duration of drug therapy for each patient was 42 days. During this time, the drug showed itself very well.
There are also results of studies of complex treatment of patients with alcohol dependence, in which Remeron was used. Reviews from doctors about the effect of the drug were also positive. In patients with withdrawal symptoms, it was possible to quickly stop panic attacks, irritability, insomnia, and normalize pulse and blood pressure. Subsequently, during the period of remission, the drug helped reduce the craving for alcohol and stabilize the mental state of patients.
Pharmacological profile
Remeron enhances central serotonergic and adrenergic transmission. The action of the active component is also to block serotonin receptors 5-HT3 and 5-HT2. Therefore, serotonergic transmission occurs through serotonin 5-HT1 receptors.
Blocks H1-histamine receptors and alpha2-adrenergic receptors.
Thanks to this, the medicine affects the body as follows:
- eliminates symptoms of depression;
- reduces anxiety and aggressiveness;
- improves mood.
The medication eliminates irritability, relieves dysphoria, relieves mental stress and gets rid of obsessive thoughts.
It has a pronounced sedative and anxiolytic effect. It is more effective for anxiety disorders and depressive states of various origins. As a result of the sedative effect, suicidal behavior is not actualized.
The antidepressant effect is observed after one to two weeks of therapy.
The drug is absorbed by the body very quickly. The highest concentration is observed after about two hours. The connection with the proteins of the active component reaches 85%. Excretion by the kidneys and intestines lasts for one to two days.
Side effects
As can be seen from the previous chapter, doctors highly value the antidepressant Remeron. Reviews from their patients are far from such unanimity. In some people, taking the drug can cause unwanted side effects. Here is a list of possible side effects listed in the instructions.
1. Digestive system:
- nausea;
- thirst;
- constipation;
- increased energy of liver transaminases;
- increased appetite;
- accumulation of body weight;
- vomit;
- abdominal pain, dry mouth.
2. From the nervous system:
- hypokinesia;
- drowsiness;
- tremor;
- lethargy;
- convulsions;
- change in mentality and mood;
- hyperkinesis;
- myoclonus;
- agitation;
- anxiety;
- depersonalization;
- apathy;
- mania;
- hallucinations;
- dizziness;
- emotional instability;
- vertigo;
- hostility;
- epileptic seizures;
- hyperesthesia.
3. Cardiovascular system:
- orthostatic hypotension;
- decrease in blood pressure.
4. Genitourinary system:
- dysmenorrhea;
- decreased potency.
5. Hematopoietic system:
- agranulocytosis;
- aplastic anemia;
- granulocytopenia;
- thrombocytopenia;
- neutropenia;
- eosinophilia.
6. Allergic reactions: urticaria.
7. Other side effects:
- edema syndrome;
- flu-like syndrome;
- myalgia;
- suffocation;
- dysuria;
- backache;
- withdrawal syndrome.
Despite all of the above, among doctors, “Remeron is considered one of the safest drugs in the category of drugs for the treatment of depression. For example, unlike tricyclic antidepressants, it does not cause “serotonin syndrome”, is practically devoid of cardiotoxicity and exhibits little anticholinergic properties such as dyspepsia and dry mouth. Some side effects (drowsiness, dizziness, increased sedation, increased appetite, etc.) are noted only at the very beginning of therapy, and subsequently disappear without a trace.
Remeron
pharmachologic effect
Remeron® (mirtazapine) is a tetracyclic antidepressant with a predominantly sedative effect.
The drug is most effective for depressive conditions with the presence in the clinical picture of symptoms such as an inability to experience pleasure and joy, loss of interest (anhedonia), psychomotor retardation, sleep disturbances (especially in the form of early awakenings) and weight loss, as well as other symptoms: suicidal thoughts and daily mood fluctuations. The antidepressant effect of Remeron® usually occurs after 1-2 weeks of treatment.
Mirtazapine is an antagonist of presynaptic α2-adrenergic receptors in the central nervous system and enhances central noradrenergic and serotonergic transmission of nerve impulses. In this case, the enhancement of serotonergic transmission is realized only through serotonin 5-HT1 receptors, since mirtazapine blocks serotonin 5-HT2 and 5-HT3 receptors. Both enantiomers of mirtazapine are believed to have antidepressant activity, the S(+) enantiomer by blocking α2-adrenergic and serotonin 5-HT2 receptors, and the R(-) enantiomer by blocking serotonin 5-HT3 receptors.
The sedative properties of mirtazapine are due to its antagonistic activity towards histamine H1 receptors. Mirtazapine is generally well tolerated. In therapeutic doses, it has virtually no anticholinergic effect and has virtually no effect on the cardiovascular system.
Pharmacokinetics
After oral administration, mirtazapine is rapidly absorbed (bioavailability about 50%), reaching Cmax in plasma in approximately 2 hours. About 85% of mirtazapine is bound to plasma proteins. The average T1/2 ranges from 20 to 40 hours (rarely up to 65 hours). A shorter T1/2 is observed in young people. The equilibrium concentration of the substance is reached after 3-4 days and subsequently does not change. In the recommended dose range, the pharmacokinetic parameters of mirtazapine have a linear relationship with the administered dose of the drug. Food intake does not affect the pharmacokinetics of the drug.
Mirtazapine is extensively metabolized and excreted in urine and feces over several days. The main pathways of its metabolism in the body are demethylation and oxidation followed by conjugation. Cytochrome P450 isoenzymes (CYP2D6 and CYP1A2) are involved in the formation of the 8-hydroxy metabolite of mirtazapine, while CYP3A4 presumably determines the formation of N-demethylated and N-oxidized metabolites. Demethylmirtazapine is pharmacologically active. Mirtazapine clearance is reduced in patients with renal or hepatic impairment.
Indications
Depression.
Dosage regimen
The tablets should be taken orally, with liquid if necessary, and swallowed without chewing.
Adults: The effective daily dose is usually between 15 and 45 mg; the initial dose is 15 or 30 mg.
Elderly: The recommended dose is the same as for adults. In elderly patients, in order to achieve a satisfactory and safe response to treatment, increasing the dose should be done under the direct supervision of a physician.
In patients with renal or hepatic impairment, the clearance of mirtazapine may be reduced. This should be taken into account when prescribing Remeron® to this category of patients.
Remeron® can be taken once a day, preferably at the same time, before bedtime. Remeron® can also be prescribed twice daily, dividing the daily dose in half (once in the morning and once at night, with the higher dose taken at night).
Treatment should, if possible, be continued for 4-6 months until the patient's symptoms disappear completely. After this, treatment can be gradually withdrawn. Mirtazapine usually begins to have its effect after 1-2 weeks of treatment. Treatment with an adequate dose should lead to a positive response within 2-4 weeks. If the response to treatment is insufficient, the dose can be increased to the maximum dose (up to 45 mg). If there is no response to treatment, treatment should be discontinued after another 2-4 weeks.
Side effect
People with depression experience a range of symptoms related to the disease, so it can sometimes be difficult to distinguish between symptoms related to the disease and symptoms caused by the drug. The following classification is used to indicate the frequency of side effects: very often (≥1/10), often (≥1/100 and ≤1/10), infrequently (>1/1000 and ≤1/100), rarely (>1/10000 and ≤1/1000), frequency not established (≤1/10000).
Blood and lymphatic system disorders:
frequency not established - bone marrow suppression (granulocytopenia, agranulocytosis, aplastic anemia and thrombocytopenia), eosinophilia.
Nervous system disorders:
very often - drowsiness (which can lead to impaired concentration), usually occurring during the first weeks of treatment. (NB dose reduction does not usually result in less sedation, but may reduce the effectiveness of the antidepressant), sedation, headache; often - lethargy, dizziness, tremor; infrequently - paresthesia, restless legs syndrome, fainting; rarely - myoclonus, very rarely - convulsions (stroke), serotonin syndrome, paresthesia of the oral mucosa.
Gastrointestinal disorders:
very often - dry mouth; often - nausea, diarrhea, vomiting; infrequently - decreased sensitivity of the oral mucosa; frequency not established - swelling of the oral mucosa.
Disorders of the skin and subcutaneous tissues:
often - skin rash.
Musculoskeletal and connective tissue disorders:
often - arthralgia, myalgia, back pain.
Endocrine system disorders:
frequency not established - impaired secretion of antidiuretic hormone;
Metabolic and nutritional disorders:
very often - increased appetite.
Vascular disorders:
often - orthostatic hypotension; infrequently - arterial hypotension.
General disorders and disorders at the injection site:
often - local edema; infrequently - fatigue.
Disorders of the liver and biliary tract:
rarely - increased serum transaminase activity.
Mental disorders:
often - unusual dreams, confusion, anxiety*, insomnia*; infrequently - nightmares, mania, agitation, hallucinations, psychomotor agitation (including akatasia and hyperkinesia); frequency not established - suicidal ideation, suicidal behavior.
Laboratory and instrumental data (based on the results of post-registration studies):
very often - weight gain.
* - Typically, when treated with antidepressants, anxiety and insomnia (which can be symptoms of depression) may develop or worsen. The development or worsening of anxiety and insomnia has been reported very rarely during treatment with Remeron®.
Contraindications
Hypersensitivity to mirtazapine or to any of the excipients.
Patients with rare hereditary problems such as lactose intolerance, lactase deficiency or glucose-galactose malabsorption should not be prescribed Remeron®. Since the safety and effectiveness of Remeron® for children under 18 years of age have not been established, the use of Remeron® for the treatment of children is not recommended.
Carefully.
Correction of the dosage regimen and regular medical monitoring are necessary for the following categories of patients:
- patients with epilepsy and organic brain lesions (during treatment with Remeron®, in rare cases, seizures may develop);
- patients with liver or kidney failure;
- patients with heart disease (conduction disorders, angina pectoris or recent myocardial infarction).
— patients with cerebrovascular diseases (including a history of ischemic stroke);
- patients with arterial hypotension and with conditions predisposing to hypotension (including dehydration and hypovolemia);
- patients who abuse drugs, are dependent on drugs that affect the central nervous system, with mania, hypomania.
Like other antidepressants, Remeron® should be used with caution in the following cases:
- urinary disturbances, incl. with prostatic hyperplasia;
- acute angle-closure glaucoma and increased intraocular pressure;
- diabetes;
- when concomitantly prescribing benzodiazepines with Remeron®.
Pregnancy and lactation
The safety of using Remeron® during pregnancy in humans has not been established, but no teratogenic effect has been detected in animals, so it can be used during pregnancy only if the benefit to the mother outweighs the potential risk to the fetus. The use of Remeron® during lactation is not recommended due to the lack of data on its excretion in human breast milk.
special instructions
When using the drug Remeron®, you should keep in mind:
- worsening of psychotic symptoms may occur when antidepressants are used to treat patients with schizophrenia or other psychotic disorders; Paranoid ideas may increase.
— the depressive phase of manic-depressive psychosis during treatment can transform into a manic phase.
- in young people (under 24 years of age) with depression and other mental disorders, antidepressants, compared with placebo, increase the risk of suicidal thoughts and suicidal behavior. Therefore, when prescribing Remeron® in young people (under 24 years of age), the risk of suicide should be weighed against the benefits of using the drug. In short-term studies, the risk of suicide did not increase in people over 24 years of age, and the risk of suicide decreased slightly in people over 65 years of age. Any depressive disorder itself increases the risk of suicide. Therefore, during treatment, the patient should be monitored to identify disturbances or changes in behavior, as well as suicidal tendencies.
- Although Remeron® is not addictive, based on post-registration experience, it appears that abrupt cessation of treatment after prolonged use can sometimes cause withdrawal symptoms. Most withdrawal reactions are mild and self-limiting. The most commonly reported withdrawal symptoms were dizziness, agitation, anxiety, headache and nausea. Although these have been reported as withdrawal symptoms, it should be understood that these symptoms may be related to an underlying medical condition. It is recommended that treatment with mirtazapine be discontinued gradually.
- Elderly patients are usually more sensitive, especially to side effects. In clinical studies of the drug Remeron®, it was not noted that in elderly patients side effects occur more often than in other age groups, but they may be more pronounced; however, data are still limited.
- if signs of jaundice appear, treatment should be interrupted.
- Patients are advised to avoid alcohol while being treated with Remeron®.
- suppression of bone marrow function, usually manifested as granulocytopenia or agranulocytosis, is rarely observed when using the drug Remeron®. Appears mostly after 4-6 weeks of treatment and is reversible after cessation of treatment. The doctor should pay close attention (and inform the patient) to symptoms such as fever, sore throat, stomatitis, and other signs of influenza-like syndrome; If such symptoms appear, you should stop treatment and do a blood test.
— based on post-registration experience, it turned out that serotonin syndrome occurs very rarely in patients receiving treatment only with Remeron®.
Impact on the ability to drive a car and operate machinery.
Remeron® may reduce concentration. During treatment with antidepressants, patients should avoid performing potentially dangerous activities that require high speed psychomotor reactions, such as driving a car or operating machinery.
Overdose
Current experience with overdose with Remeron® alone indicates that symptoms are usually mild. Central nervous system depression has been reported, accompanied by disorientation and prolonged sedation in combination with tachycardia and a slight increase or decrease in blood pressure. However, there is a potential for more severe results (including death) at doses much higher than the therapeutic dose, particularly in overdoses of multiple drugs taken at the same time. In case of overdose, symptomatic therapy should be carried out to maintain vital body functions. Activated carbon should be administered or the stomach should be rinsed.
Drug interactions
Pharmacokinetic interaction
- Mirtazapine is extensively metabolized with the participation of the CYP2D6 and CYP3A4 isoenzymes, and to a lesser extent with the participation of the CYP1A2 isoenzyme. An interaction study in healthy volunteers showed that paroxetine, an inhibitor of the CYP2D6 isoenzyme, has no effect on the pharmacokinetics of mirtazapine at steady state. Administration in combination with a strong CYP3A4 inhibitor, ketoconazole, increased the maximum plasma concentration and AUC of mirtazapine by approximately 40% and 50%, respectively. Caution should be exercised when mirtazapine is used in combination with strong CYP3A4 inhibitors, HIV protease inhibitors, azole antifungals, erythromycin, or nefazodone.
- carbamazepine and phenytoin, inducers of the CYP3A4 isoenzyme, increased the clearance of mirtazapine by approximately two times, which led to a 45-60% decrease in plasma concentrations of mirtazapine. When adding carbamazepine or another inducer of hepatic metabolism (eg, rifampicin) to mirtazapine therapy, the dose of mirtazapine should be increased if necessary. If treatment with this drug is stopped, the dose of mirtazapine may need to be reduced.
- when used in combination with cimetidine, the bioavailability of mirtazapine can increase by more than 50%. The dose of mirtazapine should be reduced if necessary when starting treatment in combination with cimetidine or increased when treatment with cimetidine is discontinued.
- In in vivo interaction studies, mirtazapine had no effect on the pharmacokinetics of risperidone or paroxetine (CYP2D6 substrate), carbamazepine (CYP3A4 substrate), amitriptyline, cimetidine or phenytoin.
- No important clinical effects or changes in pharmacokinetics were observed in humans when treated with mirtazapine in combination with lithium.
Pharmacodynamic interaction
- Mirtazapine should not be used in combination with MAO inhibitors or within two weeks after stopping treatment with a MAO inhibitor.
- Mirtazapine may enhance the sedative properties of benzodiazepines and other sedatives. Caution should be exercised when prescribing these drugs with mirtazapine.
- Mirtazapine may enhance the depressive effect of alcohol on the central nervous system. Therefore, patients should be warned to avoid drinking alcohol.
- in the case of using other serotonergic drugs (for example, selective serotonin reuptake inhibitors and venlafaxine) in combination with mirtazapine, there is a risk of interaction, which can lead to the development of serotonin syndrome. Based on post-registration experience with the drug, it turned out that serotonin syndrome occurs very rarely in patients receiving treatment with mirtazapne in combination with selective serotonin reuptake inhibitors or venlafaxine. If such a combination is considered therapeutically necessary, the dose should be adjusted cautiously and closely monitored for signs of the onset of sustained serotonergic overstimulation.
Mirtazapine 30 mg once daily caused a small but statistically significant increase in INR in subjects treated with warfarin. A more pronounced effect with a higher dose of mirtazapine cannot be excluded. It is recommended to monitor MHO in case of treatment with warfarin in combination with mirtazapine.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
At a temperature of 2-30°C, protected from light and moisture, out of reach of children
Best before date:
3 years. Do not use after the expiration date stated on the packaging.
special instructions
The antidepressant Remeron can cause increased psychotic symptoms in patients with schizophrenia. During treatment of the depressive phase of affective bipolar psychosis, an inversion of affect with the appearance of mania is possible. However, if suicidal tendencies are observed, the patient is recommended to be given a limited amount of medication. If the patient is a woman of reproductive age, then the pills are prescribed only if she is using reliable contraceptives.
Abrupt withdrawal of the drug following long-term use can cause nausea, headaches, and a general deterioration in well-being. Taking Remeron should be stopped if symptoms of any infectious diseases, jaundice, or changes in the blood appear. During the period of taking the drug, patients are instructed to be more careful when driving a car, as well as when engaging in other potentially risky types of work and activities that require speed of psychomotor reactions and increased attention span.
Overdose
Typically, an overdose of Remedon causes symptoms of mild severity: disorientation , sedation , tachycardia , hypo- or hypertension , depression of the functions of the central nervous system. If an overdose occurs with several drugs at the same time, the symptoms can be much more serious, even fatal.
Therapy is symptomatic, supportive, taking activated charcoal , gastric lavage.