Release form and composition
Fluoxetine is available in the form of hard gelatin capsules of 10 or 20 mg. Capsules have a white body and a white (10 mg) or green (20 mg) cap. Inside there are white or white with a yellowish tint granules and powder.
The drug is packaged in blisters (5 or 10 capsules each) or polymer jars (20, 30, 50 or 100 capsules each) and packaged in cardboard packs (2 or 4 blisters of 7 capsules each, 2, 3, 5 or 10 blisters of 10 capsules each or 1 can in a pack).
1 capsule contains:
- active ingredient: fluoxetine – 10 or 20 mg;
- excipients: povidone, lactose monohydrate, potato starch, calcium stearate;
- shell composition: water, titanium dioxide, gelatin; additionally for capsules 20 mg – indigo carmine, yellow iron oxide.
Contraindications
- age under 18 years;
- simultaneous use of MAO inhibitors and at least 2 weeks after their discontinuation;
- hypersensitivity to any components of the drug;
- simultaneous use of thioridazine and at least 5 weeks after its discontinuation;
- lactase deficiency, glucose-galactose malabsorption, lactose intolerance;
- simultaneous use of pimozide.
The drug is taken with caution in the following cases:
- diabetes;
- suicidal ideation;
- epilepsy (including history).
Directions for use and dosage
Fluoxetine capsules must be taken orally, regardless of food.
At the initial stage of treatment of depression, 20 mg of the drug is prescribed once a day (in the first half of the day). If necessary, the daily dose can be increased by 20 mg daily to 40–60 mg (divided into 2–3 doses). The maximum daily dose should not exceed 80 mg.
Clinical effect is usually observed 1–4 weeks after the start of therapy (in some cases longer time is required).
When treating obsessive-compulsive disorders, the recommended daily dose is from 20 to 60 mg (divided into 2-3 doses).
Patients with bulimia nervosa are recommended to take 60 mg of Fluoxetine per day (divided into 2-3 doses).
The duration of the course of therapy is determined by the doctor and can be several years. Discontinuation of the drug should be carried out gradually over 1–2 weeks.
The initial dose for elderly patients should not exceed 20 mg. If necessary, the daily dose can be increased to 40–60 mg (divided into 2–3 doses). The maximum daily dose should not exceed 80 mg.
The duration of the course of therapy is determined by the doctor, who can also adjust the dose depending on the patient’s condition.
When treating patients with low body weight, impaired renal and hepatic function, concomitant diseases, or taking other medications, it is recommended to reduce the dose and frequency of taking Fluoxetine.
Price for Fluoxetine Lannacher in pharmacies
Fluoxetine is an antidepressant.
- I’ve been taking fluoxetine for about 3 months now. For the first 2-3 weeks I really wanted to sleep, the feeling of appetite disappeared for a couple of hours after taking the capsule. Now all this has passed, I have become a calm, normal, adequate person, my appetite (unfortunately) no longer disappears, but I often want to sleep during the day (1 hour of daily rest is often enough). In general, I am very pleased with the drug, the effect is wonderful.
- I have been taking Fluoxetine for the 2nd week (2 capsules in the morning), I feel great, my mood has improved, and I have a calm attitude towards food. Personally, it helps me a lot!
- I took fluoxetine for five days. On the third day, side effects began - yawning, nausea, visual disturbances, weakness. I gave up. The doctor said that my autonomic system was swollen and that this was the first time she had seen such an effect. I haven’t taken it for five days now, the side effects persist, although they have smoothed out a little. Apparently, everything is very individual. I tolerated Coaxil well, and it is considered a much stronger drug. And on Paxil, for example, I almost threw my skates away.
- It is advisable to take it in the morning, it helps to control the day and night routine as serotonin is processed into melatonin, the sleep hormone, in the evening. The effect is observed only from about 2 packs. A normal person may not feel it at all. Everything else is subjective perception.
- I’ve been drinking for half a year, prescribed by a psychotherapist for the treatment of food addiction.... I start drinking after two weeks, the mood is better, life gets better, as soon as you stop drinking after two weeks everything comes back... and depression and gluttony..... you start drinking again - everything is fine again... I used to have PMS all the time... but with the pills I stopped noticing it...... the only thing that’s scary... is quitting drinking it... such depression begins
- I eat away all the stress. if it’s good, I eat, if it’s bad, I eat too. A friend recommended this drug, she says that the apathy has gone away and the desire to live has appeared. but I didn’t have any problems with it. She herself is a very energetic, active person. Although there are not many contraindications, I am afraid to interfere with the functioning of the brain. Withdrawal syndrome is scary. There is no depression, if you take the drug, it will appear after you stop it?!
- I started taking fluoxetine 2 weeks ago. Immediately there was an improvement - the constant tearfulness went away (sometimes tears flowed nonstop for 2-3 days), fears for my husband, myself, the future, etc. stopped, the spasmodic pain in the stomach area almost disappeared, my heart stopped fluttering like a hare's tail. BUT: an unmuffled feeling of constant uterine hunger has appeared, but there is no appetite, I eat through force, and the weight is constantly falling, and I still look like I came out of a concentration camp. But overall, an absolutely positive effect. I don’t know anything about the withdrawal effect yet, the period of use is short. Capsules made in USA
Doctors leave positive reviews about the medicine. Among other qualities, they note that although Fluoxetine affects the state of human hormones, it does not cause “addiction” and after stopping the use of the drug, the body quickly returns to normal production of the hormone that was before the start of taking the drug
Patients are also prone to positive reviews about the drug. In particular note:
- quick results from using the drug (up to 3 days)
- in addition to improved mood, a significant influx of strength is noted
- improved sleep
The cost of Fluoxetine in pharmacies depends on the form of release and the manufacturer of the drug. Prices in popular pharmacy chains range from 75 to 120 rubles (20 capsules of 20 mg each)
The drug can be found at any local pharmacy.
Side effects
- nervous system: very often – headache; often - dizziness, dysgeusia, impaired attention, tremor, drowsiness (including excessive daytime sleepiness and sedation), lethargy: infrequently - myoclonus, imbalance, ataxia, psychomotor hyperactivity, memory impairment, dyskinesia: rarely - buccoglossal syndrome, akathisia, convulsions ; unknown frequency – dysphemia, serotonin syndrome;
- mental disorders: very often - insomnia (including problems falling asleep and sleeping in the middle of the night, early morning awakenings, nightmares and other unusual dreams); often – tension, nervousness, anxiety, restlessness, decreased (even loss) libido; infrequently - euphoria, increased mood, bruxism, impaired thinking, depersonalization, impaired orgasm; rarely – panic attacks, agitation, hypomania, hallucinations; unknown frequency – suicidal behavior or thoughts;
- respiratory system: often – yawning; infrequently - the appearance of shortness of breath; rarely – pharyngitis; unknown frequency – epistaxis, inflammatory processes in the lungs;
- cardiovascular system: often – hot flashes (including hot flashes, palpitations); infrequently - decreased blood pressure; rarely – vasodilation, vasculitis;
- circulatory and lymphatic systems: very rarely - thrombocytopenia; unknown frequency - pancytopenia;
- immune system: rarely - urticaria, anaphylactic reactions, serum sickness, angioedema;
- endocrine system: unknown frequency - impaired secretion of antidiuretic hormone;
- reproductive system: often - gynecological bleeding (including uterine dysfunction, cervical bleeding, menometrorrhagia, uterine, vaginal, genital and postmenopausal bleeding), erectile dysfunction, ejaculation disorders (including ejaculatory dysfunction, delayed or absent ejaculation, premature or retrograde ejaculation); rarely – galactorrhea; unknown frequency – hyperprolactinemia, priapism;
- metabolism and nutrition: often – loss of appetite (including anorexia); rarely - hyponatremia;
- skin: often - hyperhidrosis, itching, rash (including peeling, erythema, miliaria, erythematous follicular, diffuse, macular, papular, maculopapular, morbilliform, itchy, umbilical, vesicular rash); uncommon – alopecia; rarely – purpura, ecchymosis, photosensitivity; unknown frequency - erythema multiforme with possible transition to Stevens-Johnson syndrome or Lyell's syndrome;
- organs of vision: often – blurred vision; infrequently – mydriasis;
- kidneys and urinary tract: often – increased urge to urinate (including pollakiuria); uncommon – dysuria; rarely - urinary retention; unknown frequency – urinary disturbance;
- hearing organs: unknown frequency – tinnitus;
- musculoskeletal and connective tissue: often – arthralgia; infrequently – muscle twitching; unknown frequency – myalgia;
- liver and biliary tract: unknown frequency - development of idiosyncratic hepatitis;
- general disorders and disorders at the injection site: very often - lethargy (including asthenia); often – chills, feeling of anxiety; infrequently - cold sweat, feeling of heat or cold, malaise, poor health, tendency to bruise; unknown frequency - bleeding from the mucous membranes;
- instrumental and laboratory data: often – loss of body weight; unknown frequency - impaired liver function indicators.
In most cases, the described effects are mild or moderate in severity and go away on their own, however, in some cases, side effects can be longer and more pronounced. That is why the withdrawal of Fluoxetine should be carried out gradually.
special instructions
All patients taking antidepressants should be under medical supervision to promptly identify signs of clinical deterioration, suicidal intent, and unusual behavioral changes. Such monitoring is especially important during the first few months of treatment or when reducing/increasing the dose.
During treatment with antidepressants for major depressive disorder and other disorders (mental and non-mental) in children and adults, the following side effects were observed: insomnia, panic attacks, anxiety, aggressiveness, agitation, irritability, hostility, impulsivity, akathisia, mania/hypomania. The presence of a causal relationship between these symptoms and the emergence of suicidal intentions and/or worsening depression has not been established, but there is an opinion that such phenomena may precede the appearance of suicidal intentions.
Fluoxetine has a long half-life, which should be taken into account when administered concomitantly with other drugs, as well as when replacing it with another antidepressant.
Fluoxetine binds well to plasma proteins, so the combination of this substance with other drugs that are also significantly bound to plasma proteins may affect their concentrations.
There are reports of rare cases of the development of serotonin or malignant neuroleptic syndrome associated with taking Fluoxetine (hyperthermia, muscle rigidity, catatonic manifestations, extrapyramidal neurological disorders). Most often, such effects were observed with the simultaneous use of other serotonergic drugs (including those containing L-tryptophan) and/or antipsychotics. The described symptoms can cause the development of a life-threatening condition, therefore, fluoxetine therapy should be discontinued if the following phenomena occur in combination: myoclonus, rigidity, changes in mental status (including irritability, confusion, extreme agitation with the possible development of coma and delirium, disorders of the autonomic nervous system with fluctuations in vital signs). After discontinuation of the drug, appropriate therapy is prescribed.
Taking Fluoxetine increases the risk of prolongation of the QT interval, so the drug is used with caution in the following cases:
- congenital or acquired syndrome of prolongation of the QT interval (for example, against the background of alternating use of fluoxetine with drugs that prolong the QT interval);
- increase in the duration of the QT interval in the patient's relatives;
- other clinical conditions that cause the development of arrhythmia (in particular, hypomagnesemia or hypokalemia);
- increased exposure to fluoxetine (for example, with impaired liver function).
When treated with the drug, a skin rash may appear, as well as the development of anaphylactic reactions and progressive systemic disorders (sometimes serious cases may occur involving the skin, lungs, kidneys, and liver in the pathological process). If a skin rash or other allergic reactions of unknown etiology occur, fluoxetine should be interrupted.
In rare cases, patients receiving electroconvulsive therapy may experience increased seizure duration.
When treating patients with a history of mania or hypomania, any antidepressants are prescribed with caution. If the patient is in a manic state, fluoxetine should be discontinued.
The drug is a strong inhibitor of the CYP2D6 isoenzyme, so its use may reduce the concentration of one of the most important active metabolites of tamoxifen, endoxifen. Treatment with tamoxifen cannot be combined with fluoxetine.
When treated with Fluoxetine, the development of akathisia is possible, the main manifestations of which are subjectively unpleasant sensations or restlessness, the need for constant movement, the inability to stand still or sit. Most often, such symptoms are observed in the first few weeks of treatment. When treating patients with akathisia, increasing the dose of the drug is undesirable.
While taking fluoxetine, patients may experience weight loss, which, however, is often proportional to the initial average body weight.
In some cases, fluoxetine therapy has resulted in hyponatremia. Basically, a similar effect was observed in elderly patients and people who received diuretics against the background of a decrease in circulating blood volume.
When prescribing the drug to patients with an increased risk of developing acute angle-closure glaucoma or increased intraocular pressure, the possibility of developing mydriasis must be taken into account.
In diabetes mellitus, hypoglycemia was observed in individuals receiving Fluoxetine, and hyperglycemia developed upon drug withdrawal. When treating patients in this category, dosage adjustments of insulin and/or oral hypoglycemic medications may be necessary after discontinuation of fluoxetine.
The drug may increase the tendency to bleeding (including in the gastrointestinal tract), so it should be prescribed with caution to patients who are simultaneously receiving anticoagulants and/or other drugs that alter the properties of platelets, as well as to persons with existing increased bleeding.
Fluoxetine is extensively metabolized in the liver, after which it is excreted by the kidneys. Patients with severe liver dysfunction should take lower doses or switch to taking the drug every other day.
Preclinical and clinical studies have shown that fluoxetine has a damaging effect on the genetic structure of sperm and impairs sperm quality. This effect is reversible - after discontinuation of the drug, sperm quality is restored.
Suicidal risks when taking Fluoxetine
In patients with depression, the likelihood of committing suicide attempts increases (may be relevant until the onset of stable remission). As with other antidepressants, isolated cases of suicidal thoughts and behavior have occurred during fluoxetine therapy or shortly after its completion. That is why it is necessary to ensure careful monitoring of patients at risk and to encourage them to immediately report to the doctor any unpleasant feelings or thoughts that cause concern.
Studies involving adults with major depressive disorder have identified the following risk factors for suicide in both groups (fluoxetine and placebo):
- before the start of therapy - more severe depression, presence of thoughts about death;
- during therapy – development of insomnia, worsening depression.
One of the risk factors during treatment with Fluoxetine was the development of severe psychomotor agitation (for example, panic, akathisia, agitation).
The occurrence or presence of these conditions before or during treatment is a reason for strengthening clinical monitoring or correction of the treatment.
Withdrawal syndrome
In many cases, when treatment with fluoxetine is stopped (especially when the drug is stopped abruptly), withdrawal symptoms are observed. The results of clinical studies indicate that approximately 60% of patients developed various side effects when therapy was discontinued. These data are true for both the fluoxetine group and the placebo group: in the first case, 17% of events were severe, in the second case – 12%.
The risk of withdrawal syndrome is influenced by several factors (including the duration of therapy and the rate of dose reduction). Most often, patients complain of dizziness, sensory disturbances (including paresthesia), asthenia, agitation, anxiety, sleep disturbances (including deep sleep and insomnia), nausea, vomiting, headaches, and tremor. These episodes are usually mild or moderate in severity, but in some patients they are more severe.
In most cases, the withdrawal syndrome goes away on its own within two weeks, but in some cases its manifestations may last longer (from 2 to 3 months or more). Fluoxetine withdrawal should be gradual, taking into account the patient's condition (usually this process takes 1-2 weeks).
Use during pregnancy and lactation
Epidemiological studies aimed at assessing the risk associated with fluoxetine use in early pregnancy have produced mixed results and have not provided convincing evidence of an increased risk of congenital anomalies in the fetus. At the same time, one of the meta-analyses indicates a potential risk of developing cardiovascular system defects in a child whose mother took fluoxetine during the first trimester of pregnancy.
It is also recommended to take the drug with caution in late pregnancy, since taking fluoxetine shortly before birth has in rare cases led to the development of withdrawal syndrome in the newborn (unstable temperature, apnea, respiratory distress syndrome, rapid breathing, convulsions, cyanosis, vomiting, tremor, hypoglycemia, muscle hypertension or hypotension, incessant crying, short-term increase in neuro-reflex excitability, irritability, feeding difficulties).
The effect of the drug on the process of childbirth in humans has not been studied.
Fluoxetine can pass into breast milk, so if it is prescribed during lactation, breastfeeding must be interrupted. If breastfeeding continues, the dose of the drug should be reduced.
Impact on the ability to control moving machinery and vehicles
Patients taking Fluoxetine are not recommended to engage in these activities until it is clear how the drug affects motor and mental activity.
Pharmacological properties
Pharmacodynamics
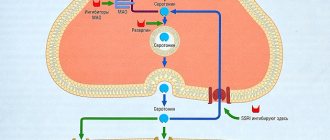
The mechanism of action of fluoxetine is based on the selective blockade of neuronal reuptake of serotonin (5-hydroxytryptamine) at the synapses of neurons in the central nervous system (CNS). As a result of suppression of serotonin reuptake, the level of this neurotransmitter increases in the synaptic cleft, and its effect on postsynaptic receptor sites is also enhanced and prolonged.
Fluoxetine has low affinity for α1, α2, and β-adrenergic, dopaminergic, muscarinic, H1-histamine, serotonin, and GABAergic receptors. The substance eliminates dysphoria, reduces anxiety, tension and fear, helps improve mood, and leads to a decrease in appetite.
Pharmacokinetics
When taken orally, the drug is well absorbed from the gastrointestinal tract (GIT). The bioavailability of the substance is not affected by food intake. The maximum concentration (Cmax) in blood plasma is observed after 6–8 hours. The binding of the substance to plasma proteins is approximately 95%, including alpha-1-acid glycoprotein and albumin. Fluoxetine has a high volume of distribution and good tissue accumulation, and easily crosses the blood-brain barrier.
Metabolic transformation occurs in the liver with the participation of the CYP2D6 isoenzyme through demethylation with the formation of the main active metabolite of the substance - norfluoxetine. Metabolites of the drug are excreted by the kidneys (80%) and intestines (15%), mainly in the form of glucuronides. After reaching equilibrium plasma concentrations, the half-life (T½) of fluoxetine can be 4-6 days, after a single dose - 1-4 days.
Drug interactions
- serotonergic agents: increased risk of developing serotonin syndrome;
- triptans: increased risk of developing arterial hypertension, serotonin syndrome, narrowing of coronary vessels;
- preparations containing St. John's wort: increased side effects;
- MAO inhibitors: increased risk of developing serotonin syndrome. A minimum of 14 days should pass between the end of taking MAO inhibitors and the start of fluoxetine. MAO inhibitors can be taken at least 5 weeks after stopping Fluoxetine;
- alcohol: experimental studies have not confirmed the fact that fluoxetine increases the concentration of alcohol in the blood and enhances its effects. At the same time, combining these substances is not recommended;
- carbamazepine, phenytoin, clozapine, haloperidol, imipramine, desipramine: changes in the concentration of these drugs in the blood, as well as isolated cases of intoxication;
- benzodiazepines: prolongation of the half-life of the latter, as well as increased concentration in the blood and increased sedative effect (when interacting with alprazolam or diazepam);
- tryptophan and lithium: development of serotonin syndrome;
- drugs affecting the coagulation system (indirect anticoagulants, acetylsalicylic acid, warfarin, non-steroidal anti-inflammatory drugs): increased bleeding (while taking oral anticoagulants), changes in the anticoagulant effect.
During pregnancy
The product is prohibited for use during pregnancy and lactation. According to research, if women in the first trimester of pregnancy received treatment with the drug, the children developed congenital anomalies in the structure of blood vessels or the heart. Taking Fluoxetine in the last trimester can lead to an increase in the duration of artificial ventilation in newborns, tube feeding, and hospitalization.
Infants develop convulsions, constant crying, hypoglycemia, nervous irritability, excitability, distress syndrome, lability of body temperature and pressure, tremor, cyanosis, vomiting, hyperreflexia, and feeding difficulties. Taking capsules during feeding is also prohibited.