| Atomoxetine | |
| Atomoxetinum | |
| Chemical compound | |
| IUPAC | (3R ) -N - methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine; ( R ) -N -methyl-3-phenyl-3-( o -toloxy)propan-1-amine |
| Gross formula | C₁₇H₂₁NO |
| Molar mass | 255.36 g/mol |
| CAS | 83015-26-3 |
| PubChem | 54841 |
| DrugBank | DB00289 |
| Classification | |
| Pharmacol. group | Adrenergic and sympathomimetics (alpha, beta) |
| ATX | N06BA09 |
| Pharmacokinetics | |
| Bioavailable | 63–94% |
| Metabolism | hepatic (CYP2D6) |
| Half-life | 5 o'clock |
| Excretion | with urine >80%, with feces <17% |
| Dosage forms | |
| capsules | |
| Method of administration | |
| orally (capsules: 10, 18, 25, 40 and 60 mg; 5, 80 and 100 mg also available in some countries) | |
| Other names | |
| Strattera | |
| Atomoxetine at Wikimedia Commons | |
Atomoxetine
((3R
)
-N
-
methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine, (
R
)
-N
-methyl-3-phenyl-3-(
o-
toloxy)propan-1-amine , trade name - "Strattera") is a medicine intended for the treatment of attention deficit hyperactivity disorder (ADD, ADHD). According to the mechanism of action, atomoxetine is a norepinephrine reuptake inhibitor (indirect centrally acting sympathomimetic).
Indications[ | ]
Strattera capsule, 60 mg
Atomoxetine is approved by the US Food and Drug Administration for the treatment of attention-deficit/hyperactivity disorder in children, adolescents and adults. Its effectiveness has not been studied in children under 6 years of age. Unlike psychostimulants traditionally prescribed for ADHD, atomoxetine is not a narcotic drug and does not have the inherent potential for abuse.[1][2] Atomoxetine has been shown in clinical trials to provide sustained, 24-hour control of ADHD symptoms in both adults and children.[3]
Atomoxetine is also used for the treatment of treatment-resistant depression, either as a stand-alone treatment or as part of a multimodal treatment regimen along with SSRIs or other medications.[4]
The therapeutic effects of atomoxetine develop gradually over at least one week. The duration of the course of use of the drug should be 6-8 weeks before deciding on its level of effectiveness. Many patients with ADHD, whose disease cannot be corrected by psychostimulants, respond to treatment with atomoxetine. Atomoxetine is also preferable in patients with a variety of mental disorders, in those who do not tolerate psychostimulants, and in patients with a history of substance abuse. Amphetamine-based psychostimulants (mixed salts of amphetamine and dextroamphetamine), pure dextroamphetamine, lisdexamfetamine, etc.) are not recommended for use in patients suffering from nervous disorders (such as facial tics, spasms, etc.). In such cases, atomoxetine is the best choice.
Atomoxetine therapy is usually started with a gradual increase in dose to minimize side effects. However, some patients are quite sensitive even to low doses. If a patient is being treated with a psychostimulant concomitantly, a gradual reduction in the dose of the psychostimulant may be necessary to prevent side effects.[5][6]
Side effects
Side effects include dry mouth, fatigue, irritability, nausea, decreased appetite, constipation, dizziness, sweating, urinary problems, sexual problems, decreased libido, urinary retention, weight changes, slower growth in children, increased heart rate and blood pressure. This clinical case was reported in the journal BMJ Case Reports by staff from the Department of Psychiatry at NHS Borders (Melrose, Scotland). We are talking about a 38-year-old woman (the authors call her Mrs. T.) who had never previously suffered from mental disorders. Mrs. T. came to see her family doctor because her daughter had recently been diagnosed with attention deficit hyperactivity disorder (ADHD), and the woman suspected that she herself also had the disorder. The doctor performed the necessary testing and agreed that Mrs. T did indeed exhibit signs of ADHD. He prescribed her the drug atomoxetine, which is commonly prescribed to treat ADHD.
Six weeks after starting the medication, the woman had to seek help from psychiatrists because she was overcome by strange ideas and began to behave abnormally. Mrs. T. developed a condition called “hallucinatory infestation”: the woman began to believe that black beetles were swarming under her skin. She, according to her, acutely felt how they were crawling there, and even saw how they crawled out from under the skin onto the surface of the hands and forearms. To get rid of the bugs, Mrs. T would scratch her skin. At the same time, it seemed to the woman that insects were emerging from spots on the kitchen wall. This caused her to make holes in the walls, which got her into trouble with the landlord. The authors of the publication described Mrs. T.’s mental state as terrible; she was very upset and agitated.
Doctors found no living creatures under the woman's skin or signs of infection, and an environmental specialist sent to her home found no insect infestation. The drug tests also came back negative, so the doctors concluded that what was happening to Mrs. T was a side effect of taking atomoxetine. After the patient stopped taking this drug and began receiving antipsychotics, her condition gradually began to improve. Six months later Mrs. T. had completely recovered. [10][11][12], aggressiveness, mania, hypomania[13].
Pharmacokinetics[ | ]
Absorption
After oral administration, atomoxetine is rapidly and almost completely absorbed, reaching maximum plasma concentration (Cmax) after approximately 1-2 hours. Atomoxetine is prescribed regardless of food intake.
Distribution
Atomoxetine is well distributed in the body. It has a high affinity for plasma proteins, primarily albumin.
Metabolism
Atomoxetine undergoes primary metabolism with the participation of the CYP2D6 isoenzyme. The main oxidized metabolite formed (4-hydroxyatomoxetine) is rapidly glucuronidated. In terms of pharmacological activity, 4-hydroxyatomoxetine is equivalent to atomoxetine, but circulates in plasma in much lower concentrations. Although 4-hydroxyatomoxetine is primarily formed by CYP2D6, in people with insufficient CYP2D6 activity, 4-hydroxyatomoxetine can be formed by some other cytochrome P450 isoenzymes, but more slowly. Atomoxetine does not inhibit or induce its own CYP2D6 cycle.
Removal
The average half-life (T1/2) of atomoxetine after oral administration is 3.6 hours in patients with pronounced metabolism and 21 hours in patients with reduced metabolism. Atomoxetine is mainly excreted in urine as 4-hydroxyatomoxetine- O
-glucuronide.
Pharmacokinetics in special clinical situations
Pharmacokinetics in children and adolescents is similar to pharmacokinetics in adults. The pharmacokinetics of atomoxetine in children under 6 years of age has not been studied.
Directions for use and doses
Orally, regardless of meals or during meals, 1 time/day, in the morning.
Treatment should be carried out under the supervision of a physician experienced in working with patients with attention deficit hyperactivity disorder.
If adverse events occur when taking the medication once a day, patients may be advised to take it twice a day, dividing the dosage into a morning dose and a late afternoon or early evening dose.
Discontinuation of the drug does not require a gradual reduction in dosage.
For children and adolescents weighing up to 70 kg, the recommended initial daily dosage is approximately 500 mcg/kg and is increased to a therapeutic daily dosage of approximately 1.2 mg/kg no earlier than after 3 days. If there is no improvement in the patient's condition, the total daily dosage can be increased to a maximum dosage of 1.8 mg/kg no earlier than 2-4 weeks after starting the medication.
The recommended maintenance dosage is approximately 1.2 mg/kg/day. The recommended maximum daily dosage is 1.8 mg/kg or 120 mg.
In children and adolescents weighing up to 70 kg, the safety of single and total daily dosages exceeding 1.8 mg/kg has not been systematically assessed.
For children and adolescents weighing more than 70 kg, as well as adults, the recommended initial daily dosage is 40 mg and increases to a therapeutic daily dosage of about 80 mg no earlier than after 3 days. If there is no improvement in the patient's condition, the total daily dosage can be increased to a maximum dosage of 120 mg no earlier than 2-4 weeks after starting the medication.
The recommended maintenance dosage is 80 mg. The recommended maximum daily dosage is 120 mg.
In children and adolescents weighing more than 70 kg and in adults, the safety of single doses greater than 120 mg and total daily doses greater than 150 mg has not been systematically assessed.
In patients with moderate hepatic impairment (Child-Pugh class B), the initial and maintenance therapeutic dose should be reduced to 50% of the usual recommended dosage. In patients with severe liver dysfunction (class C on the Child-Pugh scale), the initial and maintenance therapeutic dose should be reduced to 25% of the usual dosage.
In patients with severely impaired renal function (end-stage chronic renal failure), atomoxetine is eliminated from the body more slowly than in healthy individuals. But no differences were noted when dosage was adjusted. Therefore, Strattera can be prescribed to patients with ADHD with chronic renal failure, including end-stage, using the usual dosage regimen. Atomoxetine may cause hypertension in patients with end-stage renal disease.
Rules for using capsules
Strattera capsules are not intended to be opened. Atomoxetine causes eye irritation. If the contents of the capsule get into your eyes, rinse them immediately with water and consult a doctor. Hands and contact surfaces should be washed with water.
Pharmainamics[ | ]
Atomoxetine potently and highly selectively inhibits presynaptic membrane transporter proteins that reuptake norepinephrine, serotonin and dopamine. Coupling constants (K i
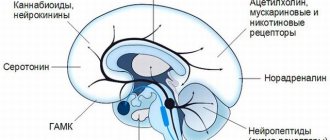
) are equal to 5, 77 and 1451 nM, respectively. In tissue microdialysis studies, atomoxetine was shown to triple norepinephrine and dopamine levels in the prefrontal cortex, but did not change dopamine levels in the striatum and nucleus accumbens.[7] Also, atomoxetine in therapeutic doses acts as an antagonist of NMDA receptors.[8] The role of such antagonism in the therapeutic profile of atomoxetine remains to be clarified, however, recent literature suggests that dysfunction of the glutamatergic system may play a role in the etiology and pathogenesis of ADHD.
Atomoxetine does not have clinically significant affinity for serotonin, acetylcholine and adrenergic receptors.[9]
Pharmacodynamics
Atomoxetine is considered a highly selective, potent inhibitor of presynaptic norepinephrine transporters. Atomoxetine has minimal affinity for other noradrenergic receptors or other neurotransmitter transporters or receptors.
Atomoxetine is not a psychostimulant and is not considered an amphetamine derivative. In clinical studies, when discontinuing the drug, there was no increase in symptoms of the disease or any adverse events associated with withdrawal syndrome.
Overdose[ | ]
Drowsiness is the most common symptom in acute or chronic overdose. Other symptoms may include agitation, hyperactivity, behavioral disturbances, and gastrointestinal symptoms. Also, an overdose sometimes causes mydriasis, due to which vision becomes blurred, tachycardia, and dry mouth. In some cases, convulsions were noted. Treatment of overdose includes gastric lavage (if a little time has passed after taking the drug) and the use of sorbents (for example, activated carbon). Atomoxetine is 98% bound to plasma proteins, so dialysis is unlikely to be beneficial.[5]
Contraindications
Taking Strattera is absolutely contraindicated for:
- serious heart lesions;
- hypersensitivity to atomoxetine
or other drug ingredients; - angle-closure glaucoma;
- conducting parallel treatment using MAO inhibitors;
- under the age of 6 years.
The drug should be prescribed with caution when:
- conditions that can cause arterial hypotension;
- tachycardia;
- arterial hypertension;
- cardiovascular diseases;
- activities associated with heavy physical exertion;
- family history of seizures or sudden cardiac death;
- joint treatment with psychostimulants;
- cerebral circulatory disorders.
Potential for abuse[ | ]
To date, the abuse potential of atomoxetine has not been widely studied. Two studies have shown that atomoxetine has a low to moderate abuse potential because it has a long titration time (meaning that the effects may not be seen until long after daily dosing). Atomoxetine does not cause significant psychostimulant effects, like most other anti-ADHD drugs. The macaques studied did not self-administer atomoxetine.[1][2] However, studies in rats, pigeons and monkeys trained to distinguish cocaine or methamphetamine from saline indicate that atomoxetine produces effects indistinguishable from low doses of cocaine or methamphetamine, but not as high as high doses of cocaine.[14][15]
In the United States, atomoxetine is not listed under the Controlled Substances Act, which typically includes substances with potential for abuse and the potential to cause psychological or physical dependence.[16]
Use for unregistered indications (off-label)[ | ]
Atomoxetine was initially studied as a treatment for depression but did not show a favorable risk/benefit ratio in clinical trials. Subsequently, Eli Lilly and Company studied atomoxetine for the treatment of ADHD. Many patients experience a significant antidepressant effect of atomoxetine (when used with other antidepressants).[17][18][19][20]
In 2007, the Journal of Clinical Psychiatry reported a double-blind, placebo-controlled, randomized study of 40 participants over 10 weeks. The effectiveness of atomoxetine in the treatment of psychogenic overeating was studied. The average dose of atomoxetine was 106 mg/day. The study found that "atomoxetine use was associated with a significantly greater rate of reduction in binge eating episodes, weight loss, and BMI." The study authors concluded that atomoxetine may be effective in the short-term treatment of binge eating disorder.[21]