Why did they talk about them?
Prions began to be studied because they are the cause of rare but very dangerous diseases in humans and animals. These include the so-called mad cow disease, sheep scrapie and, for example, lethal familial insomnia, in which a person practically stops sleeping and dies within 12–18 months due to progressive dementia. Another example of a prion disease is Creutzfeldt-Jakob disease, which leads to death within six months to a year after the first symptoms appear; the disease affects the brain, so memory and attention disorders develop, and then coordination disorder and seizures. The latter is included in the list of the most dangerous diseases in the world, because doctors simply do not know what to do with it. It all started with the mysterious kuru disease in Papua New Guinea, for which there seemed to be no reasonable explanation.
Cows and radiation
The similarity of the diseases (an unusually long incubation period and the inevitability of death, contagiousness and, of course, characteristic degeneration of the nervous tissue of the brain) made it possible to assume a common cause for them and combine them into a group of transmissible spongiform encephalopathies.
The incubation period for scrapie can last for years. But once the animal begins to show the first symptoms, you can be sure that death will occur within a few weeks.
Already in the 1960s, British scientists suggested that their source could be a protein: the mysterious infectious agent was not inactivated by a dose of radiation that was lethal even for viruses. This could be explained by the tiny size of its particles, much smaller than even viruses. The hypothesis seemed not without meaning, although it fundamentally contradicted the “basic dogma of molecular biology,” which postulated a unidirectional flow of information characteristic of all living things: from DNA through RNA to protein. Is it possible to remove two whole links from this short chain and retain many of the properties of living things? In the 1970s, Californian neurologist Stanley Prusiner began searching for the pathogen. The work did not proceed quickly: using the biological fluids of sheep infected with scrapie, scientists confirmed that the disease is inexorable and develops in every single infected mouse, although the incubation period drags on for six months, or even longer. But the results of many years of experiments turned out to be sensational: again and again, isolating a previously elusive infectious agent, scientists were convinced that it was a “naked” protein.
How they were discovered
The story of the discovery of prions resembles an adventure film with elements of science fiction: it would seem that what could be healthier than life on a beautiful island in the middle of the ocean, for example in New Guinea? But the Fore people who lived there were once struck by a disease of an unknown nature, which manifested itself as very strong shaking, preventing a person from first walking, then standing and sitting. In the end, all the patient could do was lie and tremble. Paralysis followed, and then death. For its unusual clinical manifestations, locals called the disease kuru (translated as “trembling”). To study infectious diseases, including the mysterious kuru, pediatrician and virologist Carlton Gaidushek went to New Guinea. He succeeded: in 1976, Gaidushek received the Nobel Prize “for his discoveries concerning new mechanisms of the origin and spread of infectious diseases,” sharing it with Baruch Blumberg, who discovered the hepatitis B virus.
However, Gaidushek did not discover prions themselves - he only suggested that the virus is caused by a “pathogenic particle” that is not visible under a microscope. To do this, the scientist had to spend a long time on the island, dissect a couple of hundred deceased Fore representatives and even send their organs (Gajdushek was especially interested in the brain, which in patients acquired a spongy structure) to his colleagues from different countries. The cause of the epidemic among the Fore people was ritual cannibalism: it was practiced even after the official ban by the authorities. The motives are prosaic: the Fore believed that by eating the flesh of a deceased person, his intelligence, abilities or talents would pass to them.
Zombie phenomenon
It has been shown that PrPC has a high affinity for copper ions, which may indicate its possible role in removing heavy metals that are toxic to the cell. On the other hand, the maximum concentration of PrPС is observed at the contacts between neurons of some brain regions. Cell membranes here are literally littered with this protein, which may indicate that it plays some role in the formation or stabilization of synaptic contacts. Another group of hypotheses suggests that PrPC is necessary to remove “contaminants” from the surface of cells. By binding them, the protein changes shape, and when a sufficient number of such altered proteins accumulate on the neuron membrane, a destruction mechanism is launched in it, and the cell dies.
“There are probably 20 or 30 hypotheses about exactly what task the normal cellular form of the prion protein might perform. But no specific, clear function was found for it, which is why debate arises,” Ilya Baskakov, a professor at the Maryland School of Medicine, who has devoted many years to the study of prions and prion diseases, told us. — In recent years, the possible role of PrPC in the development of the nervous system has been actively discussed. It may be necessary for new neurons to mature from stem cells - experiments have shown that if the PRNP gene encoding this protein is turned off in a stem cell, it cannot turn into a nervous one.”
The functions of the healthy protein remain unknown, but they are clearly very important: the PRNP gene is highly conserved and differs little between humans and other mammals. Theoretically, this allows the prion to be transmitted between any animal species that have PrPС - it is not without reason that spongiform encephalopathy has been recorded not only in humans, sheep and cows, but also in cats, minks, antelopes, deer and even ostriches. It is assumed that the first “zombie” protein in the population may appear by chance, as a result of an incorrectly formed spatial form of the healthy PrPC protein. This mistake, seemingly insignificant at first glance, changes everything.
Like zombies, whose ranks multiply with each bite, the infectious form of PrPSc leads to the degeneration of normal PrPS molecules into new prions - the process develops as an autocatalytic reaction, the products of which themselves accelerate it. Like zombies, prions love to “walk” in countless hordes: PrPSc particles are stacked on top of each other, forming very stable fibers, so that each end of such a formation becomes a center of attraction for more and more new prions.
“PrPSc does not occur alone, as monomers,” says Ilya Baskakov, “they exist only in the form of multimer aggregates. It has been shown that the smallest PrPSc particle can consist of about six monomers, but as they grow, they reach hundreds and thousands of units.” Having reached large sizes, the protein fiber breaks down into many new fibrils, each of which becomes the embryo of a new army of zombie prions. The cell, suppressed by accumulations of these plaques, dies, and PrPSc spread further. It is extremely difficult to destroy them: experiments have shown that prions are incredibly resistant not only to radiation, but also to heat, and even to the action of powerful cellular protease enzymes.
The incubation period for Creutzfeldt-Jakob disease is so long that some people who received the prion protein during the mad cow disease outbreak of the 1980s are only now beginning to show symptoms of the disease.
PrPSc fragments can enter the body from the outside, through biological fluids and tissues of sick animals, during certain medical procedures, and simply through food. Most of the degenerated protein particles are apparently destroyed in the gastrointestinal tract, but some manage to reach the site of action, even breaking through the blood-brain barrier that stands at the border between blood and brain tissue. The ability of PrPSc to pass through this very reliable barrier remains one of its main mysteries.
How to slow down the progression of prion disease
Until recently, it was difficult to slow down prion disease, let alone cure it.
Modern neurosurgery can alleviate degenerative complications by transplanting neurons for prion disease.
A revolutionary attempt to stop pathogenic proteins was pioneered by a team of doctors from the University of Pittsburgh. Dr. Kondziolka's team transplanted neurons in vitro (2-6 million LBS neurons) into 12 patients as part of the study. 12 months after surgery, none of the recipients had any adverse effects from the transplant, and all received new cells. In 6 patients there was a significant improvement in condition.
Several years ago, the journal Nature reported on the development of antibodies against prions in England by a team of scientists led by Simone Hawke (Imperial College). In tests on infected mice, rodents that received the antibodies were cured within a month of infection.
Doctors intend to use this opportunity to clear neurons of pathogenic proteins to treat a new variant of Creutzfeldt-Jacobs disease in humans. Further research should help improve drugs that can clear prions from the brain and its microglia.
Recently, M. Pfeiffer and several other German scientists discovered and confirmed a method to clear the brain of prions. They proved that RNA interference can successfully treat diseases by removing from the RNA cell the part responsible for the production of pathogenic proteins. This prevented them from mutating into dangerous proteins.
A similar method is the effect of artificially synthesized primers (oligonucleotides) against prions. Like RNA interference, primer design is under investigation.
Theoretically, the PrP13 peptide, similar to the structural product found in these diseases, may be promising in the treatment of diseases. The substance targets the PRNP gene, which should affect the conversion of PrPC to PrPres. The last pathogenic molecule named changes to a PrPC-like structure. The substance goes by the acronym PrP13, and animal studies have been able to prolong survival by 50-300%.
Characteristics of infections
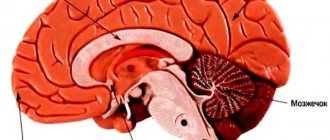
Diseases are caused by pathogens that do not contain nucleic acid. They can be sporadic, genetically determined, infectious (even iatrogenic). The presence of defective proteins causes disorders commonly called spongiform encephalopathies. These are degenerative disorders of the nervous system in which the brain gradually takes on a spongy appearance due to miniature holes due to prions killing neurons.
All infections are incurable and fatal. Today, research and testing are being conducted to explore methods of slowing the progression of prion disease.
Diagnostics
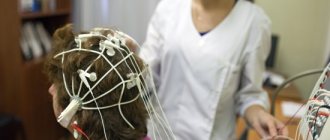
Diagnosis of prion diseases is based on the detection of cognitive disorders, their progressive development, and already at an intermediate stage of development - non-specific EEG disturbances, gradually turning into specific patterns (burst suppression pattern) with myoclonus in the clinical and EMG picture, and epileptic seizures.
Only 14-3-3 protein is present in the cerebrospinal fluid.
In sporadic Creutzfeldt-Jacobs disease, T2-weighted MRI demonstrates hyperintensity in the nucleus basalis and caudalis. In the new variant of the disease, it is present in the pulvinar thalamus and has the shape of a club.
DNA testing can help identify genetic and familial forms of the disorder, which means identifying the E200K mutation.
The clinical diagnostic conclusion is confirmed by immunohistochemical and neurohistological examination of brain tissue.
- typical neurocytic vacuolization;
- spongiomatous degeneration of the neuropil;
- loss of nerve cells;
- astrogliosis;
- presence of scrapie-associated fibrils in the affected brain.
Stop a cold without medication
Almost every month, advertisements for new innovative cold remedies appear on TV screens, one tablet of which is enough to make the disease go away. Unfortunately, no matter what marketers come up with, scientists have not yet invented such a magic pill. And if the cold has managed to take over the body, you will have to get sick for 5-7 days.
Therefore, it is better to try not to get sick at all or stop a cold at an early stage. Alas, even precautions are sometimes not enough. If the virus does enter the body, there is a chance to stop it at an early stage and prevent the disease from developing into something more serious. It is very important not to miss the first signs of illness and start acting immediately, otherwise a common cold can have consequences such as laryngitis, rhinitis, bronchitis, otitis media, sore throat and others.
Depending on the type, viruses affect the body differently, but the first signs of the disease are usually the same. These include chills, possible fever, weakness and body aches, drowsiness, decreased activity, and headache. As soon as you feel at least some of these symptoms, you need to take action.
Well-advertised medications such as Theraflu, Fervex or Coldrex will help alleviate the condition for a while and bring down a high temperature, so if you urgently need to get in shape, they are acceptable. But it must be borne in mind that these drugs do not cure, but only relieve the symptoms of the disease, while affecting the functioning of the liver, kidneys and other organs. In addition, the use of such drugs is recommended only at temperatures above 38.5 C. At low temperatures, you should not abuse antipyretic drugs; it is better to give preference to natural non-medicinal remedies.
Even with changeable spring weather, protecting yourself from illness or easing its course is a feasible task if you follow five simple tips.
- The main thing is to remember about prevention. In order to prevent pathogens from knocking you down, you must not forget about personal hygiene, simply put, wash your hands more often with warm water and soap or liquid antiseptic. Colds and flu are spread through airborne droplets, but these same droplets often remain on various surfaces that we touch with our hands and then carry into the body. In addition, it is necessary to thoroughly ventilate the room several times a day and, if possible, carry out wet cleaning.
- It happens that one of the family members or a colleague at work gets sick, and there is no escape from his sneezing. The situation is unpleasant, but in this case, disinfecting the room can help. It’s unlikely that anyone will bring a quartz lamp to work, but essential oils, especially pine and eucalyptus, will do a good job of disinfecting. Today in the pharmacy you can find not only traditional oils, but also new products that are more convenient to use - ready-made compositions, even in the form of sprays. The combination of eucalyptus, juniper, mint and other oils is more effective than one component, so a few drops or pressing the spray button is enough for the active substances to start working. They destroy pathogens that enter the air when sneezing and coughing, thereby reducing the likelihood of infection.
- At the first signs of illness, the first step is to ensure complete rest and, preferably, bed rest, and then measure the temperature. If it does not exceed 38 degrees, you should not knock it down - this is how the body fights infection. Warmth is very important at this stage. If the temperature is low and your heart allows, you can take a warm bath with an infusion of chamomile, St. John's wort or other natural antiseptic.
An alternative to a bath can be warm rubbing of the chest and feet. Probably, many remember how in childhood our mothers rubbed us with turpentine or alcohol. Now there are more effective and pleasant products, various warming gels and creams with natural ingredients, for example, essential oils, badger fat or red pepper. By the way, rubbing is indispensable if you just get your feet wet or are hypothermic - they normalize thermoregulation and improve blood circulation. You can also use good old mustard plasters, fortunately, unlike those familiar from childhood, they are now available in convenient sachets.
- Nasal congestion is often one of the first signs of illness, which unsettles even with normal general health. Advertising advises using drops that instantly “clear” your nose, but they have several disadvantages. Firstly, vasoconstrictor drops dry out the mucous membrane, secondly, their effect often lasts only for a few hours, and thirdly, they only eliminate symptoms and do not treat the cause. In addition, with prolonged use, an addictive effect occurs, and a runny nose, instead of disappearing, becomes chronic. Therefore, it is better to use drops in extreme cases, when the nose does not breathe at all.
At the initial stage, it is advisable to use essential oils that make breathing easier and also fight the cause of a runny nose. For convenience, you can use special patches soaked in essential oils. Such a patch (for example, the “Breathe” inhaler patch) is glued to clothing or any flat surface. The components included in the composition have anti-inflammatory, antiviral and antibacterial effects, eliminate congestion, but, unlike drops, do not dry out the nasal mucosa and are not addictive. One patch lasts approximately eight hours.
If you have a sore throat, do not rush to take strong medications like Hexoral or Lizobact. As in the case of a runny nose, it is better to limit yourself to natural remedies. First of all, this is rinsing with tincture of chamomile, sage and other medicinal herbs. If it is not possible to gargle, you can use natural lozenges with honey, eucalyptus and extracts of beneficial plants. They will help cope with discomfort and gently disinfect the nasopharynx.
- Another important element in effectively fighting the disease is warm, plenty of fluids. Lemon tea, which some people love, puts stress on the heart and promotes dehydration. It is best to drink berry fruit drinks and decoctions of medicinal herbs, such as chamomile and linden. Unfortunately, with the modern pace of life, not everyone has time to make fruit drinks or brew herbs, especially at work.
For this case, you can buy special drinks at the pharmacy with extracts of beneficial substances (ginger, propolis, linden, chamomile, sage, etc.), for example, “Breathe.” Typically, such drinks also contain vitamin C, which is recommended to be taken at the initial stage of the disease in order to activate the body’s protective functions. Another plus is that such drinks are easy to prepare - they come in powder form, so just pour the contents of the sachet into a cup and add water. And no fuss with herbs and roots! It is important that you need to drink little and often, and not pour 2-3 liters of liquid into yourself at once.
As for food, it should generally be limited, especially fatty and heavy foods, because the body, instead of fighting the infection, spends time digesting it. Light broths, porridges with water and steamed vegetables and fruits are enough. They will not burden the body or irritate the throat.
If you follow these tips and do not suffer from illness on your feet, but devote at least one day to your health, there is a high probability that the very next morning you will wake up as a healthy person or, at least, avoid the unpleasant complications of a cold.
Animal diseases
The risk of animal diseases for humans is very speculative (the problem is best characterized by the connection between BSE and a new variant of Creutzfeldt-Jacobs disorder). In animals, disorders are manifested by aggressiveness and impaired motor abilities:
- bovine spongiform encephalopathy (BSE);
- scrapie;
- chronic wasting disease (CWD);
- felt spongiform encephalopathy;
- transmissible encephalopathy.